Volume 18, Number 10—October 2012
Research
WU and KI Polyomaviruses in Respiratory Samples from Allogeneic Hematopoietic Cell Transplant Recipients
Cite This Article
Citation for Media
Abstract
Data are limited regarding 2 new human polyomaviruses, KI polyomavirus (KIPyV) and WU polyomavirus (WUPyV), in immunocompromised patients. We used real-time PCR to test for these and 12 respiratory viruses in 2,732 nasal wash samples collected during the first year after allogeneic hematopoietic cell transplantation from 222 patients. Specimens were collected weekly until day 100; then at least every 3 months. One year after hematopoietic cell transplantation, the cumulative incidence estimate was 26% for KIPyV and 8% for WUPyV. Age <20 years predicted detection of KIPyV (hazard ratio [HR] 4.6) and WUPyV (HR 4.4), and detection of a respiratory virus in the previous 2 weeks predicted KIPyV detection (HR 3.4). Sputum production and wheezing were associated with detection of KIPyV in the past week and WUPyV in the past month. There were no associations with polyomavirus detection and acute graft versus host disease, cytomegalovirus reactivation, neutropenia, lymphopenia, hospitalization, or death.
In 2007, two new human polyomaviruses, KI polyomavirus (KIPyV) and WU polyomavirus (WUPyV), were identified in respiratory specimens from patients with respiratory illness (1,2). Since then, KIPyV and WUPyV have been frequently detected in respiratory specimens, especially those from children with respiratory symptoms and from patients co-infected with a respiratory virus (3–11). However, KIPyV and WUPyV were detected at similar rates in specimens from symptomatic patients and in persons without respiratory symptoms (6,8,12,13), suggesting that these viruses might not cause respiratory illness in immunocompetent children.
Two other human polyomaviruses, BK and JC, cause mild or asymptomatic primary infections early in life, followed by persistent, subclinical infections in healthy persons (14–16). However, these viruses can reactivate, primarily from the kidney, bone marrow, and lymphoid tissue, and cause serious disease in immunocompromised patients (14–16). Similarly, reactivation of KIPyV and WUPyV from lymphoid tissue was described among immunosuppressed persons with AIDS, although clinical consequences of reactivation were not examined (17).
Because KIPyV and WUPyV are frequently detected in association with respiratory symptoms, inhalation is suspected as a potential route of transmission. If KIPyV and WUPyV are respiratory pathogens, they may be more likely to cause respiratory illness in immunocompromised persons, either during primary infection or reactivation. Few, mostly retrospective, studies on the prevalence of KIPyV and WUPyV in respiratory specimens have included large numbers of immunocompromised patients (18–21). No prospective data are available that comprehensively describe the incidence, symptoms, risk factors, and outcomes associated with detection of KIPyV and WUPyV in respiratory specimens from hematopoietic cell transplant (HCT) recipients. This question is particularly relevant with the increasing use of multiplex PCR panels for detection of respiratory viruses, especially in samples from immunocompromised patients.
To investigate whether respiratory detection of these new polyomaviruses is associated with specific outcomes in patients after HCT, a real-time PCR specific for KIPyV and WUPyV DNA was developed and used to examine nasal wash specimens collected prospectively from HCT recipients with and without respiratory symptoms for 1 year after transplantation. Clinical data and standardized symptom surveys obtained at each specimen collection were analyzed to determine associations between respiratory KIPyV and WUPyV detection and illness.
Patients and Collection of Specimens
Combined nasopharyngeal wash (or swab) and oropharyngeal swab samples were collected weekly beginning 1–2 weeks before transplantation until day 100; then every 1–3 months for ≤1-year after transplantation from allogeneic HCT recipients enrolled in a prospective surveillance study approved by the Institutional Review Board at Fred Hutchinson Cancer Research Center (Seattle, WA, USA) (22). Participants provided written informed consent. Additional specimens were collected when respiratory symptoms were reported. Patients had ≥1 specimen collected during January 2006–December 2007. Participants completed surveys weekly for 1 year and reported any of 11 respiratory or 4 systemic symptoms. Clinical and laboratory data were obtained from medical records.
Detection of KIPyV and WUPyV DNA and PCR Validation
An in-house, duplex, real-time TaqMan PCR was developed, which was specific for viral protein 2–3 and viral protein 1 genes of KIPyV and WUPyV, respectively. Ten microliters of extracted sample were added to the PCR master mixture containing KIPyV and WUPyV primers and probes (Table 1). Samples with PCR cycle threshold values >40 were considered negative. Viral copies per milliliter were determined by using standard curves generated by PCR amplification of 10-fold dilutions of plasmids containing amplicon sequences ranging in concentration from 10 to 1 ×107 copies/reaction. To validate this PCR, a subset of KIPyV-positive, WUPyV-positive, KIPyV-negative, and WUPyV-negative samples was blindly retested by using published assays for detection of KIPyV (23) and WUPyV (4).
The in-house PCR had a sensitivity of 5–10 DNA copies/PCR, which provided a sensitivity of 500–1,000 copies/mL. This PCR did not detect JC or BK virus DNA. A subset of 397 samples, including 31 positive for KIPyV and 27 positive for WUPyV by the PCR, was retested by using published real-time PCRs. Samples with discordant KIPyV results included 3 positive by the in-house PCR and negative by the alternate PCR (23) and 1 negative by the in-house PCR and positive by the alternate PCR. Samples with discordant WUPyV results included 4 positive by the in-house PCR and negative by the alternate PCR (4) and 1 negative by the in-house PCR and positive by the alternate PCR. Samples with discordant results had <5,000 copies/mL, indicating low levels of DNA near the assay limits of detection.
Detection of Respiratory Virus
Samples were tested for 12 respiratory viruses by using a multiplexed panel of real-time, TaqMan reverse transcription PCRs. Assays to detect respiratory syncytial virus; human metapneumovirus; influenza virus A; parainfluenza viruses 1, 2, and 3; adenoviruses; coronaviruses; rhinoviruses; and bocavirus were performed as described (24–30). Assays to detect influenza virus B and parainfluenza virus 4 were performed by using the same reagents and thermocycling conditions as the other respiratory virus assays but by substituting the specific primer and probe sets (Table 1). The sensitivity of each assay was 1,000 viral copies/mL. Throughout this report, respiratory virus refers to any of these 12 viruses.
Statistical Analysis
The probability of detecting KIPyV or WUPyV DNA in ≥1 nasal wash sample from transplantation to 1-year after transplantation was estimated by using cumulative incidence curves. Patient records were censored at 14 days past the time of last eligible respiratory sample, death, or 1 year after transplantation, whichever occurred first. Death before 1 year and within 14 days after the last sample of a patient was treated as a competing risk for detection.
The association of KIPyV and WUPyV detection with respiratory virus positivity was estimated by using a logistic regression model. Differences in KIPyV and WUPyV copies/mL according to patient age at transplantation and co-occurrence of a respiratory virus were assessed by using linear regression. For both model types, robust standard errors were calculated to account for correlation within repeated measures for the same person.
Cox regression models were fit to evaluate potential risk factors for detection of KIPyV or WUPyV in the first year after transplantation, including age, sex, disease risk (standard or high) (29,31), stem cell source, donor type, conditioning regimen (myeloablative versus nonmyeloablative), donor and recipient cytomegalovirus (CMV) serostatus, grades 2–4 graft versus host disease (GvHD), and respiratory virus detection. Donor CMV serostatus was defined as negative for cord blood recipients. Acute GvHD and respiratory virus detection were treated as time-dependent covariates; respiratory virus detection was set to 1 when ≥1 respiratory virus had been detected in the previous 2 weeks and 0 otherwise. Cox regression models were fit to evaluate risk factors for KIPyV or WUPyV detection in the first 100 days after transplantation.
CMV reactivation was modeled as a time-dependent indicator defined as any antigenemia or positive PCR value, or antigenemia >10 cells per slide or >100 copies/mL by PCR. Neutropenia was modeled as a time-dependent covariate set to 1 when the absolute neutrophil count was <500 cells/mm3. When values were missing, the indicator for the previous day was carried forward. Lymphopenia was modeled similarly with 2 thresholds, 100 and 300 cells/mm3.
We evaluated detection of KIPyV or WUPyV as predictors of clinical outcomes, including diagnosis of grades 2–4 acute GvHD, CMV reactivation, neutropenia, lymphopenia, and hospitalization within 100 days; and viral symptoms, increased liver transaminase and total bilirubin levels, and death within 1 year. For time-to-event outcomes, patient records were censored at date of last contact or death, and KIPyV and WUPyV detection were treated as time-dependent covariates in Cox regression models. Potential confounders for these models included age, sex, donor type, stem cell source, and conditioning regimen.
Outcomes with multiple occurrences over time were analyzed as functions of recent KIPyV or WUPyV detection by using logistic and linear regression models. Virus detection within the last week was defined as a positive sample at the current or last study contact and a lag time ≤14 days. Virus detection within the last month was analyzed for wheezing and cough because these symptoms may persist, and was defined as a positive sample at the current or any of the last 4 study contacts, as long as those contacts occurred within 45 days. Models were adjusted for detection of respiratory virus within the last week, day relative to transplantation, age, conditioning regimen, donor type, and acute GvHD. Measurements based on multiple patient contacts were entered as repeated measures and adjusted for possible correlation between values within a person by using generalized estimating equations.
For regression models, p values were obtained by using the Wald test; no adjustments were made for multiple comparisons. Two-sided p values <0.05 were considered significant. Analyses were performed by using SAS version 9.0 (SAS Institute, Cary, NC, USA).
Patient Characteristics and Detection of KIPyV and WUPyV
A total of 2,732 nasal wash specimens were collected from 222 eligible patients at weekly or longer intervals (median 7 days, range 4–155 days). The cohort had a median of 13 specimens per patient (range 1–30 specimens). Patients ranged in age from 9.6 months to 75.2 years (median 51.5 years) and remained in the study a median of 111 days (range 1–365 days) after HCT. A respiratory virus was detected in 17% of specimens; 51% of patients had ≥1 sample positive for any respiratory virus during their time in the study. Clinical characteristics of the cohort are provided in Table 2.
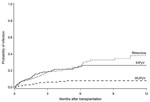
KIPyV and WUPyV DNA was detected in 203 (7%) specimens from 49 (22%) patients and in 35 (1%) specimens from 15 (7%) patients, respectively. Two patients were positive for both viruses; 1 patient was positive concurrently in the same specimen, and an additional patient was positive for 1 virus in different specimens. Among the 62 WUPyV-positive or KIPyV-positive patients, 1 patient provided only 1 specimen during the study. At 1 year after HCT, cumulative incidence estimates were 26% (95% CI 20%–33%) for KIPyV and 8% (95% CI 4%–12%) for WUPyV (Figure 1). KIPyV and WUPyV were first detected a median of 45 days (range 1–187 days) and 43 days (range 4−46 days) after transplantation, respectively. KIPyV-positive or WUPyV-positive specimens were detected in every month, and there was no apparent seasonality (Figure 2). A respiratory virus was detected in 32% of KIPyV-positive and 14% of WUPyV-positive specimens. Rhinoviruses and coronaviruses accounted for 78% of respiratory virus co-detections. Respiratory and KIPyV viruses tended to co-occur (odds ratio [OR] 2.4, 95% CI 1.2–5.1, p = 0.02).
Twenty-one (43%) KIPyV-positive and 9 (60%) WUPyV-positive patients had 1 positive specimen. Positive episodes with detection in ≥4 consecutive specimens were seen in 18 (37%) KIPyV-positive patients, including 6 with 9–19 consistently positive specimens (Figure 3, panel A) and in 2 (13%) WUPyV-positive patients, including 1 with 11 consecutive specimens (Figure 3, panel B). The maximum number (log10 copies/mL) of KIPyV and WUPyV virus per positive episode ranged from 2.55 to 10.58 (median 5.31) and 2.57–9.02 (median 3.08), respectively. Patients with >1 KIPyV-positive specimen had an average of >3 logs higher maximum viral log10 copies/mL (median 6.56) than KIPyV-positive patients with 1 positive specimen (median 2.86, p<0.001).
If we considered all positive samples, the number of KIPyV copies/mL was significantly higher in specimens positive for KIPyV and a respiratory virus than in specimens in which KIPyV was the only virus detected (medians 6.35 vs. 4.25, respectively, p<0.001). In contrast, WUPyV-positive specimens with a respiratory virus co-pathogen had significantly lower viral copy numbers than specimens positive for WUPyV virus alone (medians 2.84 vs. 3.62, respectively, p = 0.01). Patient age at transplantation was not correlated with KIPyV or WUPyV copies/mL.
Risk Factors for Detection of KIPyV and WUPyV
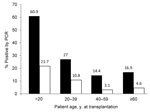
Time in study and number of specimens collected did not differ among patients according to polyomavirus detection. Age <20 years was a significant predictor of KIPyV and WUPyV detection in multivariable models (hazard ratio [HR] 4.6, 95% CI 2.5–8.6, p<0.001; HR 4.4, 95% CI 1.5–12.8, p = 0.007; respectively). All 10 patients <12 years of age and 18 (78%) of 23 patients <20 years of age were positive for KIPyV or WUPyV compared with 44 (22%) of 199 patients ≥20 years of age (Figure 4). Detection of a respiratory virus within the last 2 weeks was a significant predictor of KIPyV detection (HR 3.4, 95% CI 1.8–6.4, p<0.001) in a model adjusted for age, transplantation type, and donor type. CMV reactivation, neutropenia, and lymphopenia were not associated with detection of KIPyV and WUPyV within the first 100 days after transplantation.
Associations between KIPyV or WUPyV Detection and Symptoms
Detection of KIPyV within the past week was significantly associated with sputum production (OR 1.7, 95% CI 1.0–2.9, p = 0.04) (Table 3). WUPyV detection within the past month was significantly associated with wheezing (OR 3.1, 95% CI 1.2–8.1, p = 0.02). Limiting the analysis to 20 patients with high levels of KIPyV detection within the past week (>5 log10 copies/mL) showed a significant association with sputum production (OR 2.0, 95% CI 1.1–3.8, p = 0.03). Analysis of symptoms as a function of KIPyV positivity among 17 patients with a respiratory virus detected within the past week showed a similar association between sputum production and KIPyV detection (OR 2.7, 95% CI 1.1–6.6, p = 0.03).
Associations between KIPyV or WUPyV Detection and Clinical Outcomes
Longitudinal analyses showed significant relationships between WUPyV detection and a lower risk for lymphopenia defined by <300 cells/mm3 (OR 0.3, 95% CI 0.1–0.6, p = 0.001) and grades 2–4 acute GvHD (HR 3.1, 95% CI 1.3–7.7, p = 0.01) in a model adjusted for donor type and stem cell source among 136, 27, and 6 patients given diagnoses of grades 2, 3, and 4 acute GvHD, respectively. However, this association was based on few cases; only 5 patients had WUPyV detected before the GvHD diagnosis. No relationships between detection of KIPyV or WUPyV and CMV reactivation, risk for hospitalization ≤100 days after transplantation, and mean values for alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels were found in multivariable models.
Nineteen bronchoalveolar lavage (BAL) samples from 13 KIPyV-positive patients and 57 BAL samples from 37 KIPyV-negative and WUPyV-negative patients were tested for KIPyV and WUPyV. Collection of BAL samples occurred within 2 weeks of a positive nasal wash sample in only 4 of the KIPyV-positive patients, including 3 with collection on the same day. Only 1 of 76 BAL samples was positive for KIPyV (6.3 log10 copies/mL). This sample was collected from a 2-year-old child on the same day as the KIPyV-positive nasal wash sample (165 days after transplantation); CMV was also isolated in culture and the patient received treatment for CMV pneumonia. Of 66 deaths within 1 year of transplantation, 12 occurred in patients positive for KIPyV, 4 in patients positive for WUPyV, and 1 in a patient positive for KIPyV and WUPyV. Neither virus was associated with the 1-year mortality rate in in an adjusted model.
Our large longitudinal surveillance study provides a rigorous evaluation of KIPyV and WUPyV detection in upper respiratory tract specimens from HCT recipients. One-year cumulative incidence estimates were high, 26% and 8% for KIPyV and WUPyV, respectively, and there were prolonged episodes of detection for ≥4 weeks in 37% of patients positive for KIPyV and 13% of patients positive for WUPyV. These numbers are comparable to those of our report of rhinoviruses and coronaviruses, the most common respiratory virus types detected in the same patient population, in which we found day 100 estimates of 22% and 11%, respectively, and detection for ≥1 month in 44% and 37%, respectively (29). In comparison, cross-sectional studies testing specimens from immunocompetent children with acute respiratory tract illnesses found the prevalence of KIPyV and WUPyV ranged from 0% to 2.8% and from 2% to 7.1% (1–13,21).
A recent study of pediatric hematology/oncology patients and immunocompetent persons found a higher KIPyV mean viral load in respiratory tract specimens from the immunocompromised group, suggesting potential for increased pathogenicity in this population (21). A study in adult HCT recipients tested sequential nasopharyngeal aspirates from 31 asymptomatic patients, in which KIPyV and WUPyV were detected in 1 each of 126 samples (19). A cross-sectional study that tested specimens from 45 HCT recipients with respiratory illness detected KIPyV in 8 (17.8%), similar to our findings, but with no WUPyV detected (19,20). Respiratory viruses were often co-detected in our cohort, 32% with KIPyV and 14% with WUPyV, consistent with high rates of co-infection reported by others (1–3,6,10,21,32).
In our study, young age was a risk factor for detection of KIPyV or WUPyV, which has been reported (4,7,32,33). The seroprevalence of antibodies to KIPyV and WUPyV for children 5–20 years of age was similar to that for adults (34,35), suggesting that primary exposure occurs in childhood. We also found that detection of a respiratory virus within the previous 2 weeks was an independent risk factor for detection of KIPyV. Compared with samples in which only KIPyV DNA was detected, higher viral copy numbers of KIPyV were detected in samples also positive for a respiratory virus, perhaps because of stimulation of viral replication during a respiratory virus infection. Our current data do not provide evidence that this is a biologically meaningful difference.
Prolonged detection and younger age associated with a higher prevalence of KIPyV and WUPyV were also reported in a longitudinal study of KIPyV and WUPyV in children (33). As with BK and JC polyomaviruses, KIPyV and WUPyV might persist for life after primary infection in undetermined sites and become reactivated during periods of immune suppression. Prolonged detection episodes may represent long-term viral shedding after new acquisition of virus, especially in young patients, or reactivation or stimulation of replication of persistent virus in the respiratory tract because of immune suppression or a recent or concomitant respiratory virus infection. A site of persistence for these new polyomaviruses has not yet been identified, although human tonsils have been postulated as a reservoir and reactivation has been demonstrated in lymph nodes and spleen (17,36,37).
The longitudinal design of our study and specimen collection during symptomatic and asymptomatic periods enabled us to thoroughly evaluate symptoms associated with KIPyV and WUPyV detection in HCT recipients. We found that KIPyV detection in the past week was associated with sputum production and WUPyV detection in the past month was associated with wheezing. Limiting the analysis to patients with high viral copy numbers of KIPyV in their nasal wash sample did not provide additional associations. Likewise, co-detection of KIPyV and a respiratory virus was not associated with symptoms other than sputum production, suggesting that infection with KIPyV did not exacerbate symptoms caused by the respiratory virus. Of 50 patients who underwent bronchoscopy for workup of possible lower respiratory illness and had a BAL specimen available for testing, we detected KIPyV in 1 sample from a child who also had CMV pneumonia. Overall, our analyses provide some support for these viruses as respiratory pathogens. However, we did not observe severe respiratory illness or death that could be conclusively attributed to polyomavirus detection.
In seeking other biologically plausible and clinically relevant associations for these new viruses, we found no association of KIPyV and WUPyV detection in nasal washes with CMV reactivation, increased liver enzyme levels, hospitalization, or neutropenia in the first 100 days after transplantation. Detection of WUPyV was associated with a lower risk for lymphopenia.
We did not test other samples, such as blood, urine, or feces, for KIPyV and WUPyV DNA. Previous studies reported detection of these viruses in plasma, serum, and peripheral blood samples from patients infected with HIV-1, healthy blood donors, and children and in urine samples from children (1,2,38) and immunocompetent and immunocompromised adults (18,38). WUPyV was detected in serum and feces of children whose nasopharyngeal aspirate samples contained high viral loads (39). Both viruses were detected in fecal samples from HCT recipients (18,40), and an association was found between KIPyV and diarrhea (40). However, we did not find an association between virus and diarrhea. Another limitation is that although we report prolonged, uninterrupted KIPyV and WUPyV detection, we did not perform genetic analyses to confirm whether these detections represent the same or different viral subtypes.
In conclusion, we detected KIPyV or WUPyV in one third of allogeneic HCT recipients during the first year after transplantation. Prolonged detection episodes and high viral copy numbers in respiratory specimens were also observed. However, we did not observe many associations with acute respiratory symptoms. We found that detection of a respiratory virus was a risk factor for KIPyV detection and that concurrent detection of the polyomaviruses and respiratory viruses was common. Although it has been suggested that KIPyV and WUPyV might be major pathogens in immunosuppressed patients, we did not find a clear role for these viruses as respiratory pathogens with classic upper and lower respiratory tract symptoms. At this time, we do not recommend routine testing for these viruses in immunocompromised patients or inclusion in multiplexed respiratory virus PCR panels. Further investigations examining other specimen types, such as BAL, blood, and urine, from a larger patient cohort over a longer period of time may be necessary to elucidate the role of these viruses in highly immunocompromised patients.
Dr Kuypers is a senior research scientist in the Molecular Virology Laboratory, Department of Laboratory Medicine, University of Washington, Seattle. Her primary research interests are epidemiology and molecular detection of respiratory viruses.
Acknowledgments
We thank Cheryl Callais and other members of the study team for providing attention to study details; Chris Davis for providing database services; and Terry Stevens-Ayers, Reggie Sampoleo, and Rohit Shankar for providing laboratory expertise.
This study was supported by National Institutes of Health (grants CA 18029, HL081595, K23HL091059, L40AI071572, and K24HL093294). A.P.C. was supported by a MedImmune Pediatric Fellowship Grant Award, a Pediatric Infectious Diseases Society Fellowship Award funded by MedImmune, the Seattle Children’s Center for Clinical and Translational Research, and Clinical and Translational Science Award grant ULI RR025014.
References
- Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Identification of a third human polyomavirus. J Virol. 2007;81:4130–6. DOIPubMedGoogle Scholar
- Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. DOIPubMedGoogle Scholar
- Bialasiewicz S, Whiley DM, Lambert SB, Wang D, Nissen MD, Sloots TP. A newly reported human polyomavirus, KI virus, is present in the respiratory tract of Australian children. J Clin Virol. 2007;40:15–8. DOIPubMedGoogle Scholar
- Le BM, Demertzis LM, Wu G, Tibbets RJ, Buller R, Arens MQ, Clinical and epidemiologic characterization of WU polyomavirus infection, St. Louis, Missouri. Emerg Infect Dis. 2007;13:1936–8. DOIPubMedGoogle Scholar
- Norja P, Ubillos I, Templeton K, Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40:307–11. DOIPubMedGoogle Scholar
- Han TH, Chung JY, Koo JW, Kim SW, Hwang ES. WU polyomavirus in children with acute lower respiratory tract infections, South Korea. Emerg Infect Dis. 2007;13:1766–8. DOIPubMedGoogle Scholar
- Ren L, Gonzalez R, Xie Z, Zhang J, Liu C, Li J, WU and KI polyomavirus present in the respiratory tract of children, but not in immunocompetent adults. J Clin Virol. 2008;43:330–3. DOIPubMedGoogle Scholar
- Wattier RL, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Role of human polyomaviruses in respiratory tract disease in young children. Emerg Infect Dis. 2008;14:1766–8. DOIPubMedGoogle Scholar
- Hormozdi DJ, Arens MQ, Le BM, Buller RS, Agapov E, Storch GA. KI polyomavirus detected in respiratory tract specimens from patients in St. Louis, Missouri. Pediatr Infect Dis J. 2010;29:329–33.PubMedGoogle Scholar
- Neske F, Blessing K, Ullrich F, Prottel A, Wolfgang Kreth H, Weissbrich B. WU polyomavirus infection in children, Germany. Emerg Infect Dis. 2008;14:680–1. DOIPubMedGoogle Scholar
- Yuan XH, Jin Y, Xie ZP, Gao HC, Xu ZQ, Zheng LS, Prevalence of human KI and WU polyomaviruses in children with acute respiratory tract infection in China. J Clin Microbiol. 2008;46:3522–5. DOIPubMedGoogle Scholar
- Abed Y, Wang D, Boivin G. WU polyomavirus in children, Canada. Emerg Infect Dis. 2007;13:1939–41. DOIPubMedGoogle Scholar
- van de Pol AC, Wolfs TF, Jansen NJ, Kimpen JL, van Loon AM, Rossen JW. Human bocavirus and KI/WU polyomaviruses in pediatric intensive care patients. Emerg Infect Dis. 2009;15:454–7. DOIPubMedGoogle Scholar
- Boothpur R, Brennan DC. Human polyoma viruses and disease with emphasis on clinical BK and JC. J Clin Virol. 2010;47:306–12. DOIPubMedGoogle Scholar
- Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–37. DOIPubMedGoogle Scholar
- zur Hausen H. Novel human polyomaviruses: re-emergence of a well known virus family as possible human carcinogens. Int J Cancer. 2008;123:247–50. DOIPubMedGoogle Scholar
- Sharp CP, Norja P, Anthony I, Bell JE, Simmonds P. Reactivation and mutation of newly discovered WU, KI, and Merkel cell carcinoma polyomaviruses in immunosuppressed individuals. J Infect Dis. 2009;199:398–404. DOIPubMedGoogle Scholar
- Bialasiewicz S, Whiley DM, Lambert SB, Nissen MD, Sloots TP. Detection of BK, JC, WU, or KI polyomaviruses in faecal, urine, blood, cerebrospinal fluid and respiratory samples. J Clin Virol. 2009;45:249–54. DOIPubMedGoogle Scholar
- Debiaggi M, Canducci F, Brerra R, Sampaolo M, Marinozzi MC, Parea M, Molecular epidemiology of KI and WU polyomaviruses in infants with acute respiratory disease and in adult hematopoietic stem cell transplant recipients. J Med Virol. 2010;82:153–6. DOIPubMedGoogle Scholar
- Mourez T, Bergeron A, Ribaud P, Scieux C, de Latour RP, Tazi A, Polyomaviruses KI and WU in immunocompromised patients with respiratory disease. Emerg Infect Dis. 2009;15:107–9. DOIPubMedGoogle Scholar
- Rao S, Garcea RL, Robinson CC, Simões EA. WU and KI polyomavirus infections in pediatric hematology/oncology patients with acute respiratory tract illness. J Clin Virol. 2011;52:28–32. DOIPubMedGoogle Scholar
- Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009;11:298–303. DOIPubMedGoogle Scholar
- Bialasiewicz S, Whiley DM, Lambert SB, Gould A, Nissen MD, Sloots TP. Development and evaluation of real-time PCR assays for the detection of the newly identified KI and WU polyomaviruses. J Clin Virol. 2007;40:9–14. DOIPubMedGoogle Scholar
- Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. Human bocavirus in French children. Emerg Infect Dis. 2006;12:1251–3. DOIPubMedGoogle Scholar
- Kuypers J, Wright N, Corey L, Morrow R. Detection and quantification of human metapneumovirus in pediatric specimens by real-time RT-PCR. J Clin Virol. 2005;33:299–305. DOIPubMedGoogle Scholar
- Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–8. DOIPubMedGoogle Scholar
- Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type-specific real-time RT-PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol. 2004;31:123–9. DOIPubMedGoogle Scholar
- Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, Real-time reverse transcription–PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–9. DOIPubMedGoogle Scholar
- Milano F, Campbell AP, Guthrie KA, Kuypers J, Englund JA, Corey L, Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115:2088–94. DOIPubMedGoogle Scholar
- Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119:e70–6. DOIPubMedGoogle Scholar
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. DOIPubMedGoogle Scholar
- Abedi Kiasari B, Vallely PJ, Corless CE, Al-Hammadi M, Klapper PE. Age-related pattern of KI and WU polyomavirus infection. J Clin Virol. 2008;43:123–5. DOIPubMedGoogle Scholar
- van der Zalm MM, Rossen JW, van Ewijk BE, Wilbrink B, van Esch PC, Wolfs TF, Prevalence and pathogenicity of WU and KI polyomaviruses in children, the Netherlands. Emerg Infect Dis. 2008;14:1787–9. DOIPubMedGoogle Scholar
- Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. DOIPubMedGoogle Scholar
- Nguyen NL, Le BM, Wang D. Serologic evidence of frequent human infection with WU and KI polyomaviruses. Emerg Infect Dis. 2009;15:1199–205. DOIPubMedGoogle Scholar
- Babakir-Mina M, Ciccozzi M, Bonifacio D, Bergallo M, Costa C, Cavallo R, Identification of the novel KI and WU polyomaviruses in human tonsils. J Clin Virol. 2009;46:75–9. DOIPubMedGoogle Scholar
- Kantola K, Sadeghi M, Lahtinen A, Koskenvuo M, Aaltonen LM, Mottonen M, Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J Clin Virol. 2009;45:292–5. DOIPubMedGoogle Scholar
- Bialasiewicz S, Whiley DM, Lambert SB, Jacob K, Bletchly C, Wang D, Presence of the newly discovered human polyomaviruses KI and WU in Australian patients with acute respiratory tract infection. J Clin Virol. 2008;41:63–8. DOIPubMedGoogle Scholar
- Neske F, Blessing K, Prottel A, Ullrich F, Kreth HW, Weissbrich B. Detection of WU polyomavirus DNA by real-time PCR in nasopharyngeal aspirates, serum, and stool samples. J Clin Virol. 2009;44:115–8. DOIPubMedGoogle Scholar
- Babakir-Mina M, Ciccozzi M, Alteri C, Polchi P, Picardi A, Greco F, Excretion of the novel polyomaviruses KI and WU in the stool of patients with hematological disorders. J Med Virol. 2009;81:1668–73. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 18, Number 10—October 2012
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Jane Kuypers, Molecular Virology, University of Washington, 1616 Eastlake Ave E, Suite 320, Seattle, WA 98102, USA
Top