Volume 20, Number 12—December 2014
Dispatch
Novel Amdoparvovirus Infecting Farmed Raccoon Dogs and Arctic Foxes
Figure 1
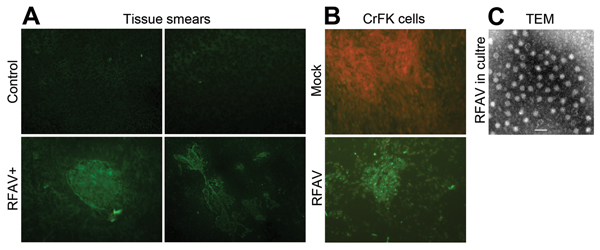
Figure 1. Detection of amdoparvovirus antigens in sick raccoon dogs and infected cells. A) Detection of amdoparvovirus antigens in tissues of sick raccoon dogs. Tissue smears, as described below, were prepared from spleen and kidney samples of sick raccoon dogs and detected with a control mouse serum or anti-AMDV-G serum by using an IFA (8). (a) Mock smear of a spleen tissue from a sick raccoon dog, detected with normal mouse serum as a primary antibody; (b) Control smear of a spleen tissue from a healthy raccoon dog; (c,d) amdoparvovirus antigen-positive smears of spleen tissue (c) and kidney tissue (d) from a sick raccoon dog, detected with anti-AMDV-G serum. Original magnification ×200. B,C) Detection of amdoparvovirus antigens and virions in infected CrFK cells. B) One milliliter filtered (0.22 μm) pathological spleen samples collected from a sick raccoon dog that had a virus titer of ≈7× 107 vgc/mL, was inoculated to confluent CrFK cells in a T25 flask. Infected cells of the fourth passage were fixed and analyzed with IFA by using anti-AMDV serum (mock- or RFAV-infected CrFK cells). C) Twenty-five milliliter supernatant (≈2× 109 vgc/mL) of RFAV(XQ-JLR)–infected CrFK cell cultures were concentrated. Virions were agglutinated with an anti-RFAV raccoon dog serum and were visualized under a transmission electron microscope (TEM). AMDV, Aleutian mink disease virus; RFAV, raccoon dog and fox amdoparvovirus; IFA, indirect immunofluorescence assay; vgc, viral genome copies. Scale bar = 50 nm.
References
- Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, The family Parvoviridae. Arch Virol. 2014;159:1239–47. DOIPubMedGoogle Scholar
- Li L, Pesavento PA, Woods L, Clifford DL, Luff J, Wang C, Novel amdovirus in gray foxes. Emerg Infect Dis. 2011;17:1876–8. DOIPubMedGoogle Scholar
- Farid AH. Aleutian mink disease virus in furbearing mammals in Nova Scotia, Canada. Acta Vet Scand. 2013;55:10–55. DOIPubMedGoogle Scholar
- Porter DD, Larsen AE, Porter HG. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973;71:331–44 .PubMedGoogle Scholar
- Cho HJ, Greenfield J. Eradication of Aleutian disease of mink by eliminating positive counterimmunoelectrophoresis test reactors. J Clin Microbiol. 1978;7:18–22 .PubMedGoogle Scholar
- Henson JB, Gorham JR, Leader RW. A field test for Aleutian disease. Natl.Fur.News. 1962;34:8–9.
- Djikeng A, Halpin R, Kuzmickas R, Depasse J, Feldblyum J, Sengamalay N, Viral genome sequencing by random priming methods. BMC Genomics. 2008;9:5–9. DOIPubMedGoogle Scholar
- Oleksiewicz MB, Costello F, Huhtanen M, Wolfinbarger JB, Alexandersen S, Bloom ME. Subcellular localization of Aleutian mink disease parvovirus proteins and DNA during permissive infection of Crandell feline kidney cells. J Virol. 1996;70:3242–7 .PubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9 . DOIPubMedGoogle Scholar