Volume 22, Number 2—February 2016
Research
Invasive Group A Streptococcus Infection among Children, Rural Kenya
Cite This Article
Citation for Media
Abstract
To determine the extent of group A Streptococcus (GAS) infections in sub-Saharan Africa and the serotypes that cause disease, we analyzed surveillance data for 64,741 hospital admissions in Kilifi, Kenya, during 1998–2011. We evaluated incidence, clinical presentations, and emm types that cause invasive GAS infection. We detected 370 cases; of the 369 for which we had data, most were skin and soft tissue infections (70%), severe pneumonia (23%), and primary bacteremia (14%). Overall case-fatality risk was 12%. Incidence of invasive GAS infection was 0.6 cases/1,000 live births among neonates, 101/100,000 person-years among children <1 year of age, and 35/100,000 among children <5 years of age. Genome sequencing identified 88 emm types. GAS causes serious disease in children in rural Kenya, especially neonates, and the causative organisms have considerable genotypic diversity. Benefit from the most advanced GAS type–specific vaccines may be limited, and efforts must be directed to protect against disease in regions of high incidence.
Worldwide, childhood deaths have decreased, largely attributable to fewer deaths from pneumonia, measles, and diarrhea (1); some of these reductions have been achieved through vaccination against common bacterial pathogens such as Streptococcus pneumoniae and Haemophilus influenzae type b (2). However, progress in reducing deaths among children has been slower in sub-Saharan Africa, where approximately half of all such deaths occur, a third during the first month of life (1). To achieve further disease reductions, it is essential to address other, potentially preventable, causes of invasive bacterial disease, such as group A Streptococcus (GAS). It is estimated that >660,000 cases of invasive GAS infection occur each year; >95% cases occur in resource-poor regions, and >160,000 patients die (3). Despite these estimates, data on invasive GAS infections in resource-poor settings are limited.
The Young Infant Study of invasive bacterial disease conducted in the late 1990s in The Gambia, Ethiopia, Papua New Guinea, and the Philippines reported GAS in 29 (17%) of 167 bacterial isolates from blood cultures and in 3 (7.5%) of 40 cerebrospinal fluid (CSF) cultures (4). Although this finding meant that GAS was the third most commonly isolated bacterium after S. pneumoniae and Staphylococcus aureus, research into associated invasive GAS infections has been limited. To our knowledge, in sub-Saharan Africa, only 1 estimate of invasive GAS incidence has been published: 29 cases/100,000 person-years (definite cases of bacteremia only) among children <5 years of age in Kenya and 96 cases/100,000 person-years among children <1 year of age (5). These incidences are higher than those reported from other resource-poor settings. Data from Fiji, in the Pacific, report an incidence of 26 cases/100,000 person-years among children <5 years and 45 cases/100,000 person-years among children <1 year of age (6). In New Caledonia, the incidence for children <5 years of age was 7 cases/100,000 person-years (7).
Vaccines for GAS are being developed; the most advanced is a 30-valent serotype-specific vaccine. Data about the emm types causing invasive GAS disease in sub-Saharan Africa are critical for assessing potential vaccine serotype coverage. Through comprehensive prospective clinical and microbiological surveillance (1998–2011), we determined incidence, clinical characteristics, and outcomes among children with invasive GAS infections in a hospital in rural Kenya. We used whole-genome sequencing to determine emm types and phylogenetic variations of invasive GAS isolates.
Study Design and Participants
Since 1998, the Kenya Medical Research Institute/Wellcome Trust Research Programme has undertaken prospective systematic clinical surveillance, including standardized clinical documentation and systematic microbiological investigation, for invasive bacterial disease among all children admitted for medical care to Kilifi County Hospital (in Kilifi, a rural area of coastal Kenya), as described elsewhere (5,8). Our observational study identified cases of invasive GAS disease during this surveillance of all children admitted to Kilifi County Hospital from August 1, 1998, through December 31, 2011. The study size was determined by admissions during the study period. The study was approved by the National Ethics Committee, Nairobi, Kenya (ERC 2144), and the Oxford Tropical Research Ethics Committee (OXTREC 151–12).
The denominator population was determined by using the Kilifi Health and Demographic Surveillance System, which covers 891 km2 surrounding the hospital and in 2011 included ≈260,000 residents (9); household enumerations are performed quarterly. We calculated the population age structure at the midpoint of the study (mid-2004) and the total number of live births.
Clinical Surveillance and Case Definitions
At the time of patient admission to the hospital, a standardized set of clinical symptoms and signs were recorded and prospectively entered into a database. At the time of patient discharge, outcome was recorded. Anthropometry for the presence of kwashiorkor (edematous malnutrition) was systematically undertaken at admission and used to define severe acute malnutrition (10). For all nonelective admissions, samples were collected for complete blood count, malaria slide, and blood culture. If clinically indicated, culture was performed for CSF, urine, and pus swab samples. Inpatient treatment was provided according to World Health Organization guidelines (10).
Starting in January 2007, in line with national guidelines, HIV testing by rapid test was offered for all children admitted. For children who had invasive GAS disease before 2007 and were not tested during our previous study of bacteremia (5), a trained counselor visited households and offered voluntary counseling and testing (9). For children who had died or were untraceable, a stored blood sample was tested by PCR for HIV. Sickle cell disease testing by electrophoresis was undertaken as clinically indicated; for children admitted with bacteremia during 1998–2008, PCR was used to retrospectively test for sickle cell disease, as described previously (11).
For this analysis, data were extracted from clinical and laboratory databases. All paper clinical records were reviewed for signs and symptoms relevant to invasive GAS disease, including the presence of pharyngitis, burns, scabies, and a vesicular rash suggestive of varicella or herpes zoster infection.
Cases of invasive GAS were defined as definite if GAS was isolated from a normally sterile site (blood, CSF, or other sterile fluid/tissue) or if necrotizing fasciitis with evidence of GAS infection was present (e.g., typical gram-positive cocci found after Gram staining or serologic testing results positive for streptococci). Cases of invasive GAS were defined as probable if any of the following were found: classic necrotizing fasciitis without microbiological confirmation; cellulitis in a patient who was moderately or severely unwell (i.e., unwell and history of parenteral receipt of antimicrobial drugs, admission to hospital, or both); microbiological confirmation (i.e., growth of GAS on culture of swab sample or serologic test results positive for streptococci); or other clinically relevant infection in a patient who is moderately or severely unwell (i.e., unwell and history of parenteral receipt of antimicrobial drugs, admission to hospital, or both), in conjunction with positive GAS culture from deep wound swab sample or biopsy sample from surgical infection site (6).
Clinical syndromes of invasive GAS disease vary. These syndromes were categorized as meningitis, severe pneumonia, skin or soft tissue infection, joint and bone infection, necrotizing fasciitis, urinary tract infection, acute glomerulonephritis, abdominal disease, endocarditis, bacteremia with no focus, and streptococcal toxic shock syndrome (Technical Appendix Table 1).
Microbiological and Molecular Methods
Blood cultures were undertaken by using the BACTEC Peds Plus system (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturers’ instructions. Positive broth cultures and CSF, urine, and surface swab samples were subcultured on 5% horse blood agar and chocolate agar. GAS isolates were identified by β-hemolysis, followed by Gram staining and catalase testing, and then grouped by latex bead agglutination. Penicillin susceptibility was tested by disk diffusion (http://www.bsac.org.uk/). Laboratory procedures were subject to internal quality control and external quality control by the UK National External Quality Assessment Service.
GAS isolates were subcultured on 5% horse blood agar from archived bacterial isolates (stored at −80°C) and transported to the Wellcome Trust Sanger Institute, Cambridge, UK. DNA was extracted by a QIAxtractor (QIAGEN, Valencia, CA, USA), and DNA quality and quantity were documented by using NanoDrop (Thermo Scientific, Waltham, MA, USA) and Qubit (Life Technologies, Carlsbad, CA, USA) techniques. Whole-genome sequences were determined from Illumina 96-plex libraries by using the HiSeq2000 sequencing platform (Illumina, San Diego, CA, USA) to generate tagged 75-bp paired-end reads. To obtain the overall population structure of the sequenced genomes, we mapped individual Illumina read pairs to the MGAS5005 (emm1) reference genome (12) by using SMALT version 0.5.8 (http://www.sanger.ac.uk/resources/software/smalt/). The average coverage of the resulting whole-genome alignment was 190×. The minimum base-call quality for identifying a single nucleotide polymorphism (SNP) was set at 50, and the minimum mapping quality for SNP calling was set at 30. SNPs called in known MGAS5005 prophage regions and repeat regions were excluded from analyses. The final genome alignment was 1,629,062 bp and comprised 125,233 SNPs. To examine the genomic relationships between the sequenced genomes, we generated a maximum-likelihood tree from the SNP alignment by using FastTree (13). Draft genome assemblies were compiled by using an iterative sequence assembly process as defined previously (14). An initial quality control screen of the short-read sequences to identify mixed isolates and low-quality sequences was determined by examining genome assembly length and SNP heterogeneity. A total of 43 (11.6%) sequences had an assembly length of >2 mega basepairs and were excluded from phylogenetic analyses because of possible contamination. The emm type and multilocus sequence type (MLST) were obtained from in-house BLAST analysis of draft genome assemblies and compared with those in centralized databases (http://www.cdc.gov/streplab/m-proteingene-typing.html, http://pubmlst.org/spyogenes/). New emm and MLST alleles were assigned by database curators. Allocation of emm cluster was derived as previously described (7). Heterogeneity observed within the typing schemes was investigated by using maximum-likelihood associations in whole-genome sequence data.
Epidemiologic Analysis
Epidemiologic analyses were undertaken by using STATA version 13 statistical software (StataCorp LP, College Station, TX, USA). Clinical characteristics of children with invasive GAS disease were tabulated, and the frequency of clinical syndromes of invasive GAS disease and associated case-fatality risks (by age group) were calculated. Incidence rates were calculated by using the invasive GAS cases resident within the Kilifi Health and Demographic Surveillance System, the age structure of the population at the study mid-point (2004), and the total number of live births. Trends in admissions were examined by using rolling averages, and a comparison between seasons (wet and dry) was made by using the Poisson distribution.
During the study, 64,761 children were admitted to the hospital with acute illness. From 370 children with invasive GAS infection, 391 GAS isolates were identified. Of these 391 isolates, 154 (39.4%) were from blood, 9 (2.3%) from CSF, 214 (54.7%) from a swab sample (wound, skin breach, or pus), 8 (2.0%) from joint aspirates, and 6 (1.5%) from urine. From 20 children, >1 GAS isolate was identified: 7 children had invasive GAS isolated from both blood and CSF; 2 children had repeat positive blood cultures; 2 children had invasive GAS isolated from blood and a swab sample; 1 child had invasive GAS isolated from CSF and a swab sample; 7 children had invasive GAS isolated from 2 swab samples; and 1 child had invasive GAS isolated from 3 swab samples. No isolates were resistant to penicillin.
Characteristics of Children and Risk Factors for Definite Invasive GAS Disease
Full clinical information was available for 369 of the 370 children: 152 children had definite and 217 had probable invasive GAS disease as defined. A total of 94 (25.5%) cases of invasive GAS were in neonates (Table 1). Among the 152 children with definite invasive GAS disease, 5 (3.3%) had burns, 4 (2.6%) had concurrent scabies, 1 (0.7%) had a vesicular rash (consistent with herpes zoster or varicella), and 2 (1.3%) had a history of trauma. Among the 217 with probable invasive GAS disease, 26 (12.0%) had burns, 3 (1.4%) had scabies, 1 (0.5%) had a vesicular rash, and 4 (1.8%) had a history of trauma (risk factors were not mutually exclusive). No reports of pharyngitis were documented for patients who had definite or probable invasive GAS disease. Among the 152 children with definite invasive GAS disease, prevalence of common risk factors for invasive bacterial disease was high: 81 (53.3%) had any risk factor; 30 (19.7%) had severe acute malnutrition, including 9 (5.9%) with kwashiorkor; 28 (18.4%) had malaria (slide positive for Plasmodium falciparum), and 24 (15.8%) had HIV infection.
Clinical Syndromes of GAS Disease and Case-Fatality Risk
Among the 369 children with invasive GAS disease, the most frequent infection was skin or soft tissue infection, occurring in 258 (69.9%); followed by severe pneumonia in 86 (23.3%), of which 59 (69%) were complicated by sepsis; then bacteremia without focus in 53 (14.4%) (Table 2). Also among these 369 children, 17 (4.6%) had bone and joint infections, 11 (3.0%) had meningitis, 6 (1.6%) had a urinary tract infection, 2 (0.5%) had acute glomerulonephritis, 1 (0.3%) had endocarditis, 1 (0.3%) had nonspecific abdominal signs, and 1 (0.3%) had necrotizing fasciitis. A total of 19 (5.1%) cases met the criteria for streptococcal toxic shock syndrome (15). Of the 369 children, 45 (12.2%) died. The case-fatality risk was highest among those with severe pneumonia (20/86, 23.3%), followed by primary bacteremia (11/53, 20.8%) and meningitis (2/11, 18.2%). Pneumonia and primary bacteremia occurred most frequently among children <1 year of age.
Incidence of Invasive GAS Disease
The minimum incidence (cases/100,000 person-years) for definite and all (definite and probable) invasive GAS disease, respectively, among children <5 years of age was 17 (95% CI 14–21) and 35 (95% CI 30–40); among children <1 year of age, incidence was 59 (95% CI 45–74) and 101 (95% CI 83–121). Among neonates, incidence (cases/1,000 live births) for definite and all invasive GAS, respectively, was 0.3 (95% CI 0.2–0.4) and 0.6 (95% CI 0.4–0.7). The incidence of death was 0.1 (95% CI 0.1–0.2) deaths per 1,000 live births (Table 3). No trend was detected in the number of cases admitted over the study period (Technical Appendix Figure 1). Invasive GAS cases occurred less frequently during the dry months across all years (December–March, 26 cases/month) than during months of the short and long rains (April–October, 33 cases/month) (p = 0.029).
Molecular Epidemiology of GAS
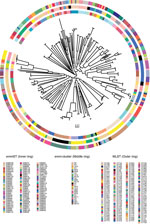
Of the 391 original GAS isolates, we retrieved 371 and generated high-quality genome sequences for 328 (Technical Appendix Table 2). From another 29 GAS isolates (combined total of 357) with lower quality genome sequences, we were able allocate an emm type. The remaining 14 samples were subsequently excluded from molecular analyses because they were not GAS or were mixed cultures, affecting accurate SNP calling (but not epidemiologic analyses because these isolates had been subcultured, stored, and then subcultured again, potentially introducing contamination). Through BLAST analysis of the 357 genome sequences against the emm typing database, we assigned 88 different emm types (97 including subtypes). Of the emm subtypes, 21 were new variants. No emm types represented >5% of the isolates studied, showing that no single emm type was predominant in the GAS population irrespective of clinical association (Figure 1, Technical Appendix Figure 2).
Of the 357 GAS isolates, we assigned an emm cluster designation to 329 on the basis of the recently described emm cluster classification scheme (16). Of the 48 emm clusters described, 24 were represented within the Kilifi invasive GAS population of isolates (Technical Appendix Table 3). Of the 140 MLSTs identified, only 24 sequence types were represented within the MLST database (78/328 strains with high-quality whole-genome sequence data). We identified 89 new allelic variants among the 7 housekeeping genes and assigned 116 new MLSTs. Crude phylogenetic analyses of the Kilifi invasive GAS population as a whole revealed a star-like topology (Figure 2) indicative of diverse core genotypes. Collectively, these data illustrate substantial heterogeneity within invasive GAS genotypes in the Kilifi population.
In terms of vaccine coverage, 99 (28%) of 357 GAS isolates are included within the current 30-valent vaccine (19), and another 104 (29%) exhibit a degree of emm cross-reactivity in vitro (Figure 1) (20). Of the remainder, 27 (8%) were not included in the vaccine and are not cross-reactive, and 127 (36%) have not yet been investigated for cross-reactivity.
Incidence of invasive GAS disease in this rural sub-Saharan African setting was strikingly high, particularly among children in the first year of life among whom GAS was a major cause of sepsis and severe pneumonia. The minimum incidence of invasive GAS infection was highest among neonates (0.6 cases/1,000 live births; more than one third of all case-patients died). Minimum incidence in the first year of life overall was also high (101 cases/100,000 person-years), twice that for Fiji, the only other resource-poor setting from which an incidence estimate is available (6). The incidence estimates presented here are probably underestimates because inclusion in the study relied on hospital admission; hence, they are referred to as minimum incidence estimates. Residents living nearer to Kilifi County Hospital are more likely to access care than those living farther from it (21), and care-seeking behavior varies (22). The incidence of invasive GAS is probably accompanied by high prevalence of the spectrum of GAS infections, including acute poststreptococcal glomerulonephritis and acute rheumatic fever, which can lead to rheumatic heart disease (23); however, data for sub-Saharan Africa are limited (24,25).
In rural Kenya, unlike in other settings, pharyngitis, varicella, and scabies did not seem to be major drivers of invasive GAS disease (23,26), and impetigo was not differentiated from skin infections. These conditions are probably underascertained because they would not in themselves result in hospital admission, and unlike most of the clinical and microbiological data (systematically sought and collected), these diagnoses relied on observations being recorded. Also, despite the high frequency of skin and soft tissue infections, we detected only 1 case of necrotizing fasciitis, which may again be underascertainment from clinical information. Invasive GAS was, however, associated with concurrent conditions driving other bacterial diseases in sub-Saharan Africa: HIV, severe acute malnutrition, and malaria (5,27,28) but not sickle cell disease (as reported elsewhere) (11,29–31).
In this study, the invasive GAS emm types and emm clusters were extremely heterogeneous and differed from those that cause disease in resource-rich settings. The presence of several S. dysgalactiae subsp. equisimilis–like emm types within a S. pyogenes genomic backbone supports previous observations of interspecies genetic transfer of emm alleles (32). The overall diversity of emm types we describe supports findings of increased heterogeneity in other resource-poor settings (33). One published study reports noninvasive GAS emm types from sub-Saharan Africa. In that study, school children in Ethiopia were investigated for GAS carriage; 43 different emm types were identified in 82 colonizing GAS isolates (34). Less than one third of emm types identified in our study were also identified in the Ethiopia study, suggesting that the pool of GAS emm types in circulation, even within neighboring countries, is larger than that described here.
Reducing the incidence of invasive GAS infection in this setting could be achieved by reducing risk factors such as severe acute malnutrition and HIV (e.g., through prevention of mother-to-child transmission), as well as by supporting antisepsis measures at delivery, including antiseptic neonatal cord care (35–37). Early and improved treatment of skin infections, including impetigo, and burns could also reduce invasive GAS disease. However, prevention through effective vaccination will probably lower disease incidence the most, as has occurred for other pathogens, such as S. pneumoniae (38) and H. influenzae type b (39). The difficulty with emm type–specific GAS vaccine approaches (19) is the heterogeneity of GAS emm types and limited data on many of the emm types identified in this study. From current information, only 57% of invasive GAS disease cases would be covered (either directly or through cross-reactivity) by the most advanced 30-valent vaccine being developed (19). Furthermore, serotype replacement could occur, as described for S. pneumoniae (40), and would require detailed surveillance.
The high incidence of invasive GAS disease in rural sub-Saharan Africa underlines the contribution of invasive bacterial disease in this region to childhood deaths, particularly among neonates and young infants; associated case-fatality risk is high. Invasive GAS may also be causing puerperal sepsis in this setting; more studies are needed. Reductions in childhood illness and death could, however, be achieved through effective GAS vaccination. Further development of GAS vaccines followed by clinical trials must be prioritized, targeted at settings with the highest disease incidence.
Dr. Seale is a research clinician trained in pediatric infectious diseases and public health. Her research interests are maternal and neonatal infections in sub-Saharan Africa.
Acknowledgments
We thank the core informatics, library, and sequencing teams at The Wellcome Sanger Institute for whole-genome sequencing; and we thank all those at the Kenya Medical Research Institute/Wellcome Trust Research Programme who were involved with surveillance at Kilifi County Hospital. We also thank and acknowledge those who set up and managed the MLST global database and the Centers for Disease Control and Prevention global emm database.
This study is published with permission from the director of the Kenya Medical Research Institute. This work was supported by the European Society for Paediatric Infectious Disease, The Wellcome Trust, UK (grant nos. 093804, 091758/Z10/Z, 098532, 083579, 077092 to A.C.S., M.R.D., T.N.W., J.A.G.S., G.D., J.B.), and KEMRI-Wellcome Trust and the National Health and Medical Research Council of Australia (grant no. 35250 to M.R.D.).
References
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40 . DOIPubMedGoogle Scholar
- Rudan I, Nair H, Marusic A, Campbell H. Reducing mortality from childhood pneumonia and diarrhoea: the leading priority is also the greatest opportunity. J Glob Health. 2013;3:010101 . DOIPubMedGoogle Scholar
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. DOIPubMedGoogle Scholar
- The WHO Young Infants Study Group. Bacterial etiology of serious infections in young infants in developing countries: results of a multicentre study. Pediatr Infect Dis. 1999;18(Suppl):S17–22. DOIGoogle Scholar
- Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. DOIPubMedGoogle Scholar
- Steer AC, Jenney A, Kado J, Good MF, Batzloff M, Waqatakirewa L, Prospective surveillance of invasive group A streptococcal disease, Fiji, 2005–2007. Emerg Infect Dis. 2009;15:216–22.PubMedGoogle Scholar
- Baroux N, D'Ortenzio E, Amedeo N, Baker C, Ali Alsuwayyid B, Dupont-Rouzeyrol M, The emm-cluster typing system for group A Streptococcus identifies epidemiologic similarities across the Pacific region. Clin Infect Dis. 2014;59:e84–92. DOIPubMedGoogle Scholar
- Aiken AM, Mturi N, Njuguna P, Mohammed S, Berkley JA, Mwangi I, Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet. 2011;378:2021–7. DOIPubMedGoogle Scholar
- Scott JA, Bauni E, Moisi JC, Ojal J, Gatakaa H, Nyundo C, Profile: the Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol. 2012;41:650–7. DOIPubMedGoogle Scholar
- World Health Organization. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2nd ed. Geneva: The Organization; 2013.
- Williams TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case–control study. Lancet. 2009;374:1364–70. DOIPubMedGoogle Scholar
- Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192:771–82. DOIPubMedGoogle Scholar
- Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–50. DOIPubMedGoogle Scholar
- Chewapreecha C, Harris SR, Croucher NJ, Turner C, Marttinen P, Cheng L, Dense genomic sampling identifies highways of pneumococcal recombination. Nat Genet. 2014;46:305–9. DOIPubMedGoogle Scholar
- The Working Group on Severe Streptococcal Infections. Defining the group A streptococcal toxic shock syndrome. Rationale and consensus definition. JAMA. 1993;269:390–1. DOIPubMedGoogle Scholar
- Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–8.PubMedGoogle Scholar
- Sanderson-Smith M, De Oliveira DM, Guglielmini J, McMillan DJ, Vu T, Holien JK, A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis. 2014;210:1325–38.PubMedGoogle Scholar
- Enright MC, Spratt BG, Kalia A, Cross JH, Bessen DE. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect Immun. 2001;69:2416–27. DOIPubMedGoogle Scholar
- Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein–based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine. 2011;29:8175–8. DOIPubMedGoogle Scholar
- Dale JB, Penfound TA, Tamboura B, Sow SO, Nataro JP, Tapia M, Potential coverage of a multivalent M protein–based group A streptococcal vaccine. Vaccine. 2013;31:1576–81. DOIPubMedGoogle Scholar
- Moïsi JC, Nokes DJ, Gatakaa H, Williams TN, Bauni E, Levine OS, Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi District, Kenya. Bull World Health Organ. 2011;89:102–11. DOIPubMedGoogle Scholar
- Herbert HK, Lee AC, Chandran A, Rudan I, Baqui AH. Care seeking for neonatal illness in low- and middle-income countries: a systematic review. PLoS Med. 2012;9:e1001183. DOIPubMedGoogle Scholar
- Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin Microbiol Rev. 2014;27:264–301 .DOIPubMedGoogle Scholar
- Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. DOIPubMedGoogle Scholar
- Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–41. DOIPubMedGoogle Scholar
- Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australas J Dermatol. 2000;41:139–43, quiz 44–5. DOIPubMedGoogle Scholar
- Scott JA, Berkley JA, Mwangi I, Ochola L, Uyoga S, Macharia A, Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case–control study and a longitudinal study. Lancet. 2011;378:1316–23. DOIPubMedGoogle Scholar
- Berkley JA, Bejon P, Mwangi T, Gwer S, Maitland K, Williams TN, HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis. 2009;49:336–43. DOIPubMedGoogle Scholar
- Suara RO. Group A beta-haemolytic streptococcal acute chest event in a child with sickle cell anaemia. Ann Trop Paediatr. 2001;21:175–8. DOIPubMedGoogle Scholar
- Aken’Ova YA. Bakare RA, Okunade MA, Olaniyi J. Bacterial causes of acute osteomyelitis in sickle cell anaemia: changing infection profile. West Afr J Med. 1995;14:255–8 .PubMedGoogle Scholar
- LeBlanc W, Salah H, Khakoo Y. Group A beta-hemolytic streptococcal bacteremia in a patient with sickle cell anemia on penicillin prophylaxis. J Natl Med Assoc. 1995;87:347–8 .PubMedGoogle Scholar
- McNeilly CL, McMillan DJ. Horizontal gene transfer and recombination in Streptococcus dysgalactiae subsp. equisimilis. Front Microbiol. 2014.5:676. PMID:25566202DOIGoogle Scholar
- Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–6. DOIPubMedGoogle Scholar
- Abdissa A, Asrat D, Kronvall G, Shittu B, Achiko D, Zeidan M, High diversity of group A streptococcal emm types among healthy schoolchildren in Ethiopia. Clin Infect Dis. 2006;42:1362–7. DOIPubMedGoogle Scholar
- Arifeen SE, Mullany LC, Shah R, Mannan I, Rahman SM, Talukder MR, The effect of cord cleansing with chlorhexidine on neonatal mortality in rural Bangladesh: a community-based, cluster-randomised trial. Lancet. 2012;379:1022–8. DOIPubMedGoogle Scholar
- Mullany LC, Darmstadt GL, Khatry SK, Katz J, LeClerq SC, Shrestha S, Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet. 2006;367:910–8. DOIPubMedGoogle Scholar
- Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq SC, Impact of newborn skin-cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119:e330–40. DOIPubMedGoogle Scholar
- Murray J, Agocs M, Serhan F, Singh S, Deloria-Knoll M, O’Brien K, Global invasive bacterial vaccine-preventable diseases surveillance—2008–2014. MMWR Morb Mortal Wkly Rep. 2014;63:1159–62 .PubMedGoogle Scholar
- Adegbola RA, Secka O, Lahai G, Lloyd-Evans N, Njie A, Usen S, Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144–50. DOIPubMedGoogle Scholar
- Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 22, Number 2—February 2016
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Anna C. Seale, KEMRI-Wellcome Trust, PO Box 230, Kilifi 80108, Kenya
Top