Volume 24, Number 3—March 2018
CME ACTIVITY - Synopsis
Epidemiology of Recurrent Hand, Foot and Mouth Disease, China, 2008–2015
Cite This Article
Citation for Media
Introduction

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; and (4) view/print certificate.
Release date: February 16, 2018; Expiration date: February 16, 2019
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe the epidemiologic features of recurrent hand-foot-and-mouth disease (HFMD), using national surveillance data during 2008–2015 in mainland China
• Identify the virologic features of recurrent HFMD
• Determine the clinical implications of the epidemiologic and virologic features of recurrent HFMD
CME Editor
Kristina B. Clark, PhD, Copyeditor, Emerging Infectious Diseases. Disclosure: Kristina B. Clark, PhD, has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed the following relevant financial relationships: owns stock, stock options, or bonds from Pfizer.
Authors
Disclosures: Jiao Huang, PhD; Qiaohong Liao, MD; Zhaorui Chang, MD; Peng Wu, PhD; Fengfeng Liu, MD; Yu Li, MSc; Li Luo, MD; Shuanbao Yu, PhD; and Sheng Wei, PhD, have disclosed no relevant financial relationships. Mong How Ooi, MBBS, MRCP, PhD, has disclosed the following relevant financial relationships: served as a speaker or a member of a speakers bureau for Sanofi Pasteur. Benjamin J. Cowling, PhD, has disclosed the following relevant financial relationships: received grants for clinical research from Sanofi Pasteur. Hongjie Yu, MD, PhD, has disclosed the following relevant financial relationships: received grants for clinical research from: Shenzhen Sanofi Pasteur Biological Products; GlaxoSmithKline (China) Investment Co.; YiChang HEC ChangJiang Pharmaceutical Co.
Abstract
Using China’s national surveillance data on hand, foot and mouth disease (HFMD) for 2008–2015, we described the epidemiologic and virologic features of recurrent HFMD. A total of 398,010 patients had HFMD recurrence; 1,767 patients had 1,814 cases of recurrent laboratory-confirmed HFMD: 99 reinfections of enterovirus A71 (EV-A71) with EV-A71, 45 of coxsackievirus A16 (CV-A16) with CV-A16, 364 of other enteroviruses with other enteroviruses, 383 of EV-A71 with CV-A16 and CV-A16 with EV-A71, and 923 of EV-A71 or CV-A16 with other enteroviruses and other enteroviruses with EV-A71 or CV-A16. The probability of HFMD recurrence was 1.9% at 12 months, 3.3% at 24 months, 3.9% at 36 months, and 4.0% at 38.8 months after the primary episode. HFMD severity was not associated with recurrent episodes or time interval between episodes. Elucidation of the mechanism underlying HFMD recurrence with the same enterovirus serotype and confirmation that HFMD recurrence is not associated with disease severity is needed.
Hand, foot and mouth disease (HFMD) is a common childhood infectious disease that is mainly caused by enterovirus A71 (EV-A71), coxsackievirus A16 (CV-A16), and CV-A6 (1). Most HFMD patients exhibit a benign, self-limiting illness characterized by skin eruptions on the hands, feet, or buttocks and ulcers or blisters in the mouth with or without fever (2). However, some patients develop neurologic or cardiopulmonary complications or die (3,4). In the past 2 decades, outbreaks of HFMD have been documented in countries of the Western Pacific, including Malaysia, Japan, Singapore, Vietnam, and Cambodia (5–9). In China, HFMD has been prevalent since 2007. During 2008–2015, ≈13 million HFMD cases were reported, including 123,261 severe cases and 3,322 deaths in 31 provinces of mainland China.
Three inactivated monovalent EV-A71 vaccines have been licensed in China. Phase 3 clinical trials proved these vaccines had high efficacy (90.0%–97.4%) against EV-A71–associated HFMD (10,11) but did not confer cross-protection for HFMD caused by non–EV-A71 enteroviruses (11). A natural infection with EV-A71 also confers no or only short-term (<2 months duration) cross-protection against CV-A16–associated illness (12,13). Because of this limited cross-protection from infections of different enterovirus serotypes, multiple HFMD episodes can occur in a single person. Although observational studies indicate that the antibody response induced by the EV-A71 vaccine could last >2 years, reinfection with an enterovirus of the same serotype is still possible because the immunity induced by a natural enterovirus infection might not be lifelong (14). We accessed the national surveillance data for HFMD diagnosed during 2008–2015 in China, in an attempt to describe the epidemiologic features of patients with recurrent HFMD and examine the relationship between disease severity and HFMD recurrence.
Data Sources
As described previously (1), HFMD cases were reported voluntarily to the Chinese Center for Disease Control and Prevention (China CDC) during January 1, 2008–May 1, 2008, and starting May 2, 2008, cases were mandatorily reported online to China CDC within 24 hours after diagnosis. We collected information on basic demographics (name, sex, national identification number, date of birth, home address, name of either of the patient’s parents, contact telephone number); case classification (probable or confirmed); disease severity (severe or mild); date of illness onset, diagnosis, and death (if applicable); and enterovirus serotype (for confirmed cases). For virologic surveillance, clinical specimens were collected from a subsample of cases from each province and tested by PCR with primers and probes for panenterovirus, EV-A71, and CV-A16. We assumed that the enterovirus identified in HFMD patient samples was the causative enterovirus of the HFMD episode.
We included the HFMD surveillance data of 29 provinces of mainland China collected during January 1, 2008–December 31, 2015. We excluded data from Hunan and Hubei Provinces from this study because (since 2010 for Hunan Province and 2012 for Hubei Province) most of the hospitals in these provinces reported EV-A71 infection on the basis of EV-A71 IgM antibody detection assays, which are unreliable tests (15–17).
Case Definitions
We defined a probable HFMD patient as a patient who had rashes on the hands, feet, mouth, or buttocks and ulcers or vesicles in the mouth with or without fever. We defined a laboratory-confirmed patient as a probable patient with laboratory evidence of infection with EV-A71, CV-A16, or other enteroviruses. The diagnostic tests used for enterovirus detection were reverse transcription PCR and real-time reverse transcription PCR. Patients were classified as having severe HFMD if they had any complications (i.e., aseptic meningitis, brainstem encephalitis, encephalitis, encephalomyelitis, acute flaccid paralysis or autonomic nervous system dysregulation, pulmonary edema, pulmonary hemorrhage, or cardiorespiratory failure). Otherwise, patients were classified as having mild HFMD.
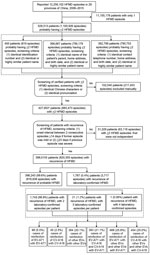
We identified patients with >2 episodes of HFMD reported in the national HFMD surveillance system by matching records using any of the following 3 screening criteria: 1) having identical identification number and identical or highly similar patient name; 2) having identical patient’s parent name, home address, and birth date and identical or highly similar patient name; and 3) having identical contact telephone number, home address, and birth date and identical or highly similar patient name (Technical Appendix). We considered patients to have recurrent HFMD if they experienced >2 independent episodes of HFMD. We defined independent episodes as consecutive episodes separated by an interval of >14 days if the previous episode was mild and >23 days if the previous episode was severe. We had estimated the time intervals defining 2 independent episodes by adding the longest duration of HFMD reported (7 days for mild illness and 16 days for severe illness) (18–21) plus the longest incubation period reported (7 days) (4,22–26). We classified patients with >2 independent episodes of laboratory-confirmed HFMD as having recurrent laboratory-confirmed HFMD. Otherwise, we classified patients as having recurrent probable HFMD. When counting the number of cases of reinfection with EV-A71, CV-A16, or other enteroviruses (Figure 1; Table 1), we considered any 2 laboratory-confirmed episodes as 1 case of reinfection; therefore, we classified patients with 3 laboratory-confirmed HFMD episodes as having 3 cases of reinfection (i.e., we grouped episodes 1 and 2, 1 and 3, and 2 and 3 together) and 4 laboratory-confirmed HFMD episodes as having 6 cases of reinfection (i.e., we grouped episodes 1 and 2, 1 and 3, 1 and 4, 2 and 3, 2 and 4, and 3 and 4 together).
Data Analysis
We used medians and interquartile ranges (IQRs) to describe continuous variables and numbers and percentages to summarize categorical variables. We used logistic regression with the forward stepwise selection approach to explore the association between HFMD recurrence and severe disease. We denoted the results as odds ratios (ORs) with 95% CIs. All statistical tests were 2-sided, and we considered an α of 0.05 statistically significant.
We defined the probability of HFMD recurrence as the probability of occurrence of HFMD among children who previously had HFMD and estimated recurrence using survival analysis (the Kaplan-Meier method). To calculate the probability of HFMD recurrence, we took only the event of interest into account and censored other events at the end of observation. When estimating the probability of HFMD recurrence, we enrolled all patients with recurrent HFMD (whether probable or laboratory-confirmed) in the analysis. We censored patients with only 1 HFMD episode. When estimating the probability of reinfection after EV-A71 with EV-A71, we included only the case-patients with a primary episode of EV-A71 infection who were later reinfected with EV-A71. We censored case-patients who had just 1 infection with EV-A71 (i.e., case-patients who were infected with EV-A71 then infected with CV-A16 or other enteroviruses, case-patients who were infected with EV-A71 then had probable HFMD, and case-patients with a single episode of EV-A71 infection). We used similar analyses to estimate the probability of reinfection with the same serotype for other serotypes of enterovirus.
We also conducted a sensitivity analysis to account for the uncertainty of the intervals used to define 2 independent HFMD episodes; in this analysis, we used 16 days (for previous mild episodes) and 61 days (for previous severe episodes) as cutoff values, which were derived from another investigation conducted in China that also investigated HFMD recurrence (27). We conducted data cleaning and analyses using R Project version 3.2.5 (http://cran.r-project.org) and ArcGIS 10.2 (http://www.esri.com/arcgis/about-arcgis). This study was approved by the ethics review committees of China CDC (Beijing, China).
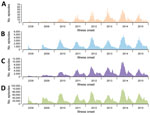
During 2008–2015, a total of 12,256,102 HFMD episodes occurring in 29 provinces of China were reported to the China CDC surveillance system. When using >14-day and >23-day intervals for defining independent infections, 398,010 patients (having 820,355 [7%] episodes) were identified as having recurrent HFMD, of which 1,767 (0.4%) patients (having 3,717 episodes) had recurrent laboratory-confirmed HFMD (Figure 1). The number of patients with recurrent HFMD was similar when we repeated this analysis using the 16-day and 61-day cutoff values in the sensitivity analysis, indicating that our estimation of HFMD recurrence was robust (Technical Appendix Figure). Compared with patients with only 1 laboratory-confirmed HFMD episode, patients with recurrent laboratory-confirmed HFMD had a similar seasonal pattern, presenting semiannual peaks of activity with a major peak in the spring and early summer (April–June) followed by a smaller peak in autumn (September–October) (Figure 2, panels A, B). Similar seasonality was also observed for patients with a single episode of and recurrent probable HFMD (Figure 2, panels C, D).
We next focused on analyzing the 1,767 patients with recurrent laboratory-confirmed HFMD. During the study period, 90.3% (1,595) of these patients had 2 episodes and 9.7% (172) had >2 episodes: 161 (9.1%) patients had 3 episodes and 11 (0.6%) patients had 4 episodes. In total, 9% (154/1,767) of the patients with recurrent laboratory-confirmed HFMD still had >1 episode of probable HFMD, and 1,613 patients had only episodes of laboratory-confirmed HFMD. Of the 157 (8.9%) patients with >1 severe HFMD episode (183 total severe episodes), 26 patients (20 with 2 episodes, 3 with 3 episodes, and 3 with 4 episodes) experienced 2 severe HFMD episodes. A total of 1,814 cases of recurrence occurred among the 1,767 patients with recurrent HFMD. Only 144 (8%) of these 1,814 cases involved reinfection with an enterovirus of the same serotype: 99 (5.5%) with EV-A71 and 45 (2.5%) with CV-A16 (Figure 1). Most recurrent HFMD cases were caused by enteroviruses of different serotypes. Of the 1,767 patients, 5 (0.3%) were found to have an interval of <20 days between consecutive HFMD episodes: 2 patients who were reinfected with enteroviruses of different serotypes and 3 patients who were reinfected with enteroviruses of the same serotype.
Demographic Characteristics
The median ages of patients with recurrent laboratory-confirmed HFMD were 22.6 (IQR 14.2–34.0) months for the first episode and 36.5 (IQR 25.7–48.7) months for the second episode. Younger children had more episodes of recurrent laboratory-confirmed HFMD (p<0.001) and recurrent probable HFMD (p = 0.001) (Technical Appendix Table 1). Few patients (1.5%) had their first episode of HFMD after 5 years of age. Approximately two thirds (68% or 1,208) of the patients affected were boys, and 41% were residents of rural areas. The demographic characteristics age, sex, and rural residence and the frequency of episodes were similar among patients with recurrent laboratory-confirmed HFMD, regardless of the enterovirus serotypes of reinfections (Table 1).
Geographic Distribution
Patients with recurrent laboratory-confirmed HFMD were observed in all of the 29 provinces of China we examined except Tibet. The number of recurrent laboratory-confirmed HFMD cases varied substantially, ranging from 7 cases in Qinghai Province to 658 cases in Guangdong Province. Half of the cases with recurrent laboratory-confirmed HFMD were reported in 3 provinces: Guangdong (658 cases), Yunnan (153 cases), and Sichuan (107 cases) (Figure 3).
Probability of HFMD Recurrence
Patients in this cohort were under observation for a median of 38.0 (range 0–97.4) months after their first HFMD diagnosis (Technical Appendix Table 2). The recurrent episode occurred 0.5–93.4 (median 11.7) months after the primary HFMD episode in patients with recurrence of probable HFMD and 0.5–62.1 (median 12.0) months in patients with recurrence of laboratory-confirmed HFMD. The probability of HFMD recurrence was 1.9% at 12 months, 3.3% at 24 months, and 3.9% at 36 months; however, recurrence remained at 4.0% at 38.8 months after the primary episode of HFMD (Figure 4, panel A). For patients with primary EV-A71 infections, the probability of reinfection with CV-A16 (0.11% [95% CI 0.10%–0.13%]) or other enteroviruses (0.14% [95% CI 0.13%–0.16%]) was higher than that of reinfection with EV-A71 (0.05% [95% CI 0.04%–0.07%]; p<0.001) (Figure 4, panel B). For patients with primary CV-A16 infections, the probability of reinfection with EV-A71 (0.18% [95% CI 0.15%–0.20%]) or other enteroviruses (0.21% [95% CI 0.18%–0.23%]) was higher than that of reinfection with CV-A16 (0.04% [95% CI 0.03%–0.05%]; p<0.001) (Figure 4, panel C). These findings suggest that risk for reinfection with different enterovirus serotypes might be higher than that for reinfection with identical enterovirus serotypes.
Relationship between HFMD Recurrence and Severe Illness
Unsurprisingly, we found that illness severity was inversely associated with age and onset-to-diagnosis interval. EV-A71 infections (OR 7.2, 95% CI 4.0–13.0) and other enterovirus infections (OR 2.7, 95% CI 1.5–5.0) were more severe than CV-A16 infections. Patients who lived in urban areas also had increased risk for severe illness (OR 1.8, 95% CI 1.3–2.5). After adjusting for these risk factors, recurrent HFMD episodes were not found to be associated with illness severity, which means the second and third HFMD episodes did not appear to be more or less severe than the first episode. In addition, the interval between the 2 episodes was not related to disease severity of the latter episode (OR 0.97, 95% CI 0.95–1.01) (Table 2).
During 2008–2015, we found that 398,010 HFMD patients with >2 episodes (a total of ≈820,000 episodes) were reported among children in China; 1,767 of these recurrences were laboratory-confirmed. The patients who were reinfected with different enterovirus serotypes had similar age, sex, residence, and frequency of episodes. Recurrence of HFMD mainly occurred 0–38.8 months after the primary episode, with a recurrence probability of 4% at 38.8 months. Reinfection with a different enterovirus serotype was more likely than reinfection with an identical enterovirus serotype. The severity of HFMD was not associated with recurrent infections or the time interval between HFMD episodes.
In a report on a phase 3 clinical trial, an EV-A71 neutralizing antibody titer of 1:16 was associated with protection against EV-A71–associated HFMD (28). In addition, EV-A71 was observed to confer cross-neutralization activity against major global EV-A71 genotypes (A, B1, B3–B5, C1–C5), although the degree of cross-neutralization varied (29–31). In an EV-A71 vaccine study, participants were observed for only 2 years, but results suggested that the vaccine can provide protection against EV-A71–associated HFMD for >2 years (14). Infection with enteroviruses seems to confer lifelong immunity to HFMD, given that adult cases are rare (1). The reasons underlying the cases of HFMD recurrence caused by reinfections with the same serotype, which have a 12-month median interval to reinfection, are not clear. Measles has been deemed to provide long-lasting protection against reinfection. However, measles reinfections have occurred in vaccinated and presumptively immune persons, either because of insufficient primary antigenic stimulation or inadequate duration of antibody response (32). Study results have suggested the involvement of multiple cellular deficiencies, including low memory B-cell count, reduced polyclonal naive and memory T-cell responses, and suboptimal antigen-presenting cell responses, in children with low vaccine responses (33,34). Agammaglobulinemia is another condition of immunodeficiency associated with recurrent infections (35). In patients with influenza, suboptimal immune responses after the primary infection led to the failure to generate a protective immune response that could have prevented reinfection (36). Children with immature immunity or immunodeficiency (37) probably are not able to induce sufficient immune responses (or might induce low-level serologic responses that wane rapidly) after infection with EV-A71 or CV-A16; thus, these children are likely susceptible to reinfection with an enterovirus of the same serotype as their primary episode. Another possibility (though less likely) is that high neutralizing antibody titers might not protect some persons from illnesses induced by enteroviruses. Further investigations are needed to provide a scientific explanation.
Even though the genotypes of EV-A71 and CV-A16 were not available in this study, previous studies have shown that the predominant EV-A71 and CV-A16 genotypes circulating in China have been consistent. Phylogenetic analysis of viral protein 1 gene sequences revealed that the EV-A71 genotype circulating in China since 2008 has been C4 (38–42); hence, the monovalent EV-A71 vaccines licensed in China were designed to target the C4 genotype. B1 has been reported to be the predominant genotype of CV-A16 circulating in China (40–43). Therefore, we reasonably conclude that in our cohort HFMD recurrences involving reinfections with enteroviruses of the same serotype were also highly likely reinfections with the same genotype.
Recurrent laboratory-confirmed HFMD was largely (at least 72% of cases) attributable to different serotypes of enterovirus; thus, undoubtedly hundreds of thousands of patients with HFMD recurrence with different serotypes should have occurred, given clinical samples were collected from only 4.2% of the patients with probable HFMD episodes for virologic diagnosis. Our results support the notion that limited or no cross-protection against different serotypes of enterovirus occurs after natural infection, which is consistent with observations from the EV-A71 vaccine study (12,14) and a modeling study of natural infections (13). Antibody-dependent enhancement, which has been commonly seen in dengue, was also observed in EV-A71 and CV-B3 infections in mouse models; the severity of the subsequent episode of infection was enhanced by a subneutralizing level of antibody after primary infection (44,45). However, we did not observe that HFMD recurrence or the interval between successive episodes had any effect on disease severity in humans. It seems that antibody-dependent enhancement does not happen in human infections with enterovirus, further implying that no or short-term cross-reactivity develops for different serotypes of enterovirus.
Three monovalent EV-A71 vaccines are administered in China (46). Our study indicates that children who receive an EV-A71 vaccine can still develop HFMD after vaccination, which is a challenge for monovalent EV-A71 vaccines. Even though the EV-A71 vaccine protects against >90% of the EV-A71 infections that occur in children, children still face the risk for infection with other enterovirus serotypes after vaccination. Hence, public health authorities should inform parents and caregivers about the risk for the development of HFMD after EV-A71 vaccination, and multivalent vaccines for HFMD (e.g., EV-A71 combined with other prevalent circulating serotypes CV-A16 and CV-A6) are needed for the HFMD epidemic.
This study has several limitations. First, the burden of HFMD recurrence was underestimated because the disease is substantially underreported in the surveillance system (16%–36% estimated) (47) and the observation period for assessing recurrence was insufficient, especially among patients identified in more recent years. Although the recurrence of HFMD is rarely reported in other countries (48,49), our study suggests that it is not uncommon. Second, because clinical samples were not collected from all patients during each HFMD episode and tested, we could not determine the real number of recurrent HFMD cases; thus, the probabilities of reinfection with an identical enterovirus serotype (i.e., EV-A71 and CV-A16) we calculated might be underestimated. It is not favorable to estimate the number of patients with reinfections of the same serotype because only a small proportion (4.2%) of HFMD episodes have been tested for enterovirus diagnosis, although mathematical modeling methods could be used to solve this problem. This topic requires further exploration. Third, we were unable to describe the features of patients with reinfection of non–EV-A71 and non–CV-A16 serotypes. Fourth, the short interval between consecutive episodes in some patients suggests the potential for co-infections rather than reinfections; thus, co-infections might have occurred and caused a slight overestimation of the recurrence rate for HFMD. However, patients with short intervals between consecutive episodes accounted for a small proportion of the patients with recurrent HFMD, so the effect that co-infections played is relatively limited.
In conclusion, our 8-year surveillance study indicates a high burden of HFMD recurrence among children in China and shows that each episode of recurrent HFMD is more likely caused by a different enterovirus serotype than that of the primary episode (both for patients with EV-A71 and CV-A16 primary infections). Further studies in which virologic diagnosis is performed for all HFMD episodes are needed to better quantify the probability of HFMD recurrence and probability of reinfection by enterovirus serotype, including non–EV-A71 and non–CV-A16 serotypes. Further investigations are also warranted to elucidate the mechanism underlying HFMD recurrences resulting from reinfections with enteroviruses of the same serotype; the protective antibody levels for EV-A71, CV-A16, and other enterovirus serotypes; and the duration of immunity and cross-immunity between serotypes. Finally, more work is needed to study the effect of HFMD recurrence on disease severity, even though no association was observed in this patient cohort.
Ms. Huang is a doctoral candidate at Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Her research interests are focused on the epidemiology of HFMD.
Acknowledgments
We thank staff members at the Bureau of Disease Control and Prevention, the National Health and Family Planning Commission of China, and provincial and local departments of health for providing assistance in coordinating the data collection. We also thank staff members at the county, prefecture, and provincial levels of CDCs and hospitals for data collection.
This study was supported by grants from the National Science Fund for Distinguished Young Scholars (no. 81525023 to H.Y.), the National Natural Science Foundation of China (no. 81473031 to. H.Y.), the Li Ka Shing Oxford Global Health Programme (no. B9RST00-B900.57 to H.Y.), and TOTAL Foundation (no. 2015-099 to H.Y.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, et al. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis. 2014;14:308–18. DOIPubMedGoogle Scholar
- Tseng FC, Huang HC, Chi CY, Lin TL, Liu CC, Jian JW, et al.; CDC-Taiwan Virology Reference Laboratories and Sentinel Physician Network. Epidemiological survey of enterovirus infections occurring in Taiwan between 2000 and 2005: analysis of sentinel physician surveillance data. J Med Virol. 2007;79:1850–60. DOIPubMedGoogle Scholar
- Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341:936–42. DOIPubMedGoogle Scholar
- Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–105. DOIPubMedGoogle Scholar
- Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, et al.; Outbreak Study Group. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis. 2000;31:678–83. DOIPubMedGoogle Scholar
- Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, Totsuka A, et al. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol. 2002;46:621–7. DOIPubMedGoogle Scholar
- Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, et al.; Taiwan Enterovirus Epidemic Working Group. An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med. 1999;341:929–35. DOIPubMedGoogle Scholar
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis. 2003;9:78–85. DOIPubMedGoogle Scholar
- Van Tu P, Thao NTT, Perera D, Truong KH, Tien NTK, Thuong TC, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733–41. DOIPubMedGoogle Scholar
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370:829–37. DOIPubMedGoogle Scholar
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–32. DOIPubMedGoogle Scholar
- Huang WC, Huang LM, Kao CL, Lu CY, Shao PL, Cheng AL, et al. Seroprevalence of enterovirus 71 and no evidence of crossprotection of enterovirus 71 antibody against the other enteroviruses in kindergarten children in Taipei city. J Microbiol Immunol Infect. 2012;45:96–101. DOIPubMedGoogle Scholar
- Takahashi S, Liao Q, Van Boeckel TP, Xing W, Sun J, Hsiao VY, et al. Hand, foot, and mouth disease in China: modeling epidemic dynamics of enterovirus serotypes and implications for vaccination. PLoS Med. 2016;13:e1001958. DOIPubMedGoogle Scholar
- Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, et al. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15:129–37. DOIPubMedGoogle Scholar
- Yu N, Guo M, He SJ, Pan YX, Chen XX, Ding XX, et al. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J. 2012;9:12. DOIPubMedGoogle Scholar
- Xu F, Yan Q, Wang H, Niu J, Li L, Zhu F, et al. Performance of detecting IgM antibodies against enterovirus 71 for early diagnosis. PLoS One. 2010;5:e11388. DOIPubMedGoogle Scholar
- Zhang J, Weng Z, Du H, Xu F, He S, He D, et al. Development and evaluation of rapid point-of-care tests for detection of Enterovirus 71 and Coxsackievirus A16 specific immunoglublin M antibodies. J Virol Methods. 2016;231:44–7. DOIPubMedGoogle Scholar
- Wong SS, Yip CC, Lau SK, Yuen KY. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol Infect. 2010;138:1071–89. DOIPubMedGoogle Scholar
- Wang Y, Feng Z, Yang Y, Self S, Gao Y, Longini IM, et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology. 2011;22:781–92. DOIPubMedGoogle Scholar
- Chia MY, Chiang PS, Chung WY, Luo ST, Lee MS. Epidemiology of enterovirus 71 infections in Taiwan. Pediatr Neonatol. 2014;55:243–9. DOIPubMedGoogle Scholar
- Zhou H, Guo SZ, Zhou H, Zhu YF, Zhang LJ, Zhang W. Clinical characteristics of hand, foot and mouth disease in Harbin and the prediction of severe cases. Chin Med J (Engl). 2012;125:1261–5.PubMedGoogle Scholar
- Liao Y, Ouyang R, Wang J, Xu B. A study of spatiotemporal delay in hand, foot and mouth disease in response to weather variations based on SVD: a case study in Shandong Province, China. BMC Public Health. 2015;15:71. DOIPubMedGoogle Scholar
- Onozuka D, Hashizume M. The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ. 2011;410-411:119–25. DOIPubMedGoogle Scholar
- Hii YL, Rocklöv J, Ng N. Short term effects of weather on hand, foot and mouth disease. PLoS One. 2011;6:e16796. DOIPubMedGoogle Scholar
- Wu H, Wang H, Wang Q, Xin Q, Lin H. The effect of meteorological factors on adolescent hand, foot, and mouth disease and associated effect modifiers. Glob Health Action. 2014;7:24664. DOIPubMedGoogle Scholar
- Yi L, Lu J, Kung HF, He ML. The virology and developments toward control of human enterovirus 71. Crit Rev Microbiol. 2011;37:313–27. DOIPubMedGoogle Scholar
- Sun L, Wand J. System review of hand, foot, and mouth disease recurrence [in Chinese]. Anhui J Prev Med. 2013;19:5.
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–28. DOIPubMedGoogle Scholar
- Mao Q, Cheng T, Zhu F, Li J, Wang Y, Li Y, et al. The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy chinese infants and children. PLoS One. 2013;8:e79599. DOIPubMedGoogle Scholar
- Liu L, Mo Z, Liang Z, Zhang Y, Li R, Ong KC, et al. Immunity and clinical efficacy of an inactivated enterovirus 71 vaccine in healthy Chinese children: a report of further observations. BMC Med. 2015;13:226. DOIPubMedGoogle Scholar
- Chou AH, Liu CC, Chang JY, Jiang R, Hsieh YC, Tsao A, et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One. 2013;8:e79783. DOIPubMedGoogle Scholar
- Linnemann CC, Hegg ME, Rotte TC, Phair JP, Schiff GM. Measles IgM response during reinfection of previously vaccinated children. J Pediatr. 1973;82:798–801. DOIPubMedGoogle Scholar
- Surendran N, Nicolosi T, Kaur R, Morris M, Pichichero M. Prospective study of the innate cellular immune response in low vaccine responder children. Innate Immun. 2017;23:89–96. DOIPubMedGoogle Scholar
- Pichichero ME. Challenges in vaccination of neonates, infants and young children. Vaccine. 2014;32:3886–94. DOIPubMedGoogle Scholar
- Sugimoto M, Takeichi T, Muramatsu H, Kojima D, Osada Y, Kono M, et al. Recurrent cellulitis caused by Helicobacter cinaedi in a patient with X-linked agammaglobulinaemia. Acta Derm Venereol. 2017;97:277–8. DOIPubMedGoogle Scholar
- Trakulsrichai S, Watcharananan SP, Chantratita W. Influenza A (H1N1) 2009 reinfection in Thailand. J Infect Public Health. 2012;5:211–4. DOIPubMedGoogle Scholar
- Koch RM, Kox M, de Jonge MI, van der Hoeven JG, Ferwerda G, Pickkers P. Patterns in bacterial- and viral-induced immunosuppression and secondary infections in the ICU. Shock. 2017;47:5–12. DOIPubMedGoogle Scholar
- Zhang Y, Wang J, Guo W, Wang H, Zhu S, Wang D, et al. Emergence and transmission pathways of rapidly evolving evolutionary branch C4a strains of human enterovirus 71 in the Central Plain of China. PLoS One. 2011;6:e27895. DOIPubMedGoogle Scholar
- Li J, Sun Y, Du Y, Yan Y, Huo D, Liu Y, et al. Characterization of coxsackievirus A6- and enterovirus 71-associated hand foot and mouth disease in Beijing, China, from 2013 to 2015. Front Microbiol. 2016;7:391.PubMedGoogle Scholar
- He SZ, Chen MY, Xu XR, Yan Q, Niu JJ, Wu WH, et al. Epidemics and aetiology of hand, foot and mouth disease in Xiamen, China, from 2008 to 2015. Epidemiol Infect. 2017;145:1865–74. DOIPubMedGoogle Scholar
- Huang X, Wei H, Wu S, Du Y, Liu L, Su J, et al. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Henan, China, 2008-2013. Sci Rep. 2015;5:8904. DOIPubMedGoogle Scholar
- Xu M, Su L, Cao L, Zhong H, Dong N, Xu J. Enterovirus genotypes causing hand foot and mouth disease in Shanghai, China: a molecular epidemiological analysis. BMC Infect Dis. 2013;13:489. DOIPubMedGoogle Scholar
- Yong W, Qiao M, Shi L, Wang X, Wang Y, Du X, et al. Genetic characteristics of coxsackievirus A16 associated with hand, foot, and mouth disease in Nanjing, China. J Infect Dev Ctries. 2016;10:168–75. DOIPubMedGoogle Scholar
- Han JF, Cao RY, Deng YQ, Tian X, Jiang T, Qin ED, et al. Antibody dependent enhancement infection of enterovirus 71 in vitro and in vivo. Virol J. 2011;8:106. DOIPubMedGoogle Scholar
- Kishimoto C, Kurokawa M, Ochiai H. Antibody-mediated immune enhancement in coxsackievirus B3 myocarditis. J Mol Cell Cardiol. 2002;34:1227–38. DOIPubMedGoogle Scholar
- China Food and Drug Administration. Announcement on licensed drugs approved by China Food and Drug Administration. 2016 [cited 2017 May 20]. http://app2.sfda.gov.cn/datasearchp/gzcxSearch.do?searchcx=71&optionType=V1&formRender=cx
- Wu JT, Jit M, Zheng Y, Leung K, Xing W, Yang J, et al. Routine pediatric enterovirus 71 vaccination in China: a cost-effectiveness analysis. PLoS Med. 2016;13:e1001975. DOIPubMedGoogle Scholar
- Dias EDM, Dias M. Recurring hand foot mouth disease in a child. Ann Trop Med Public Health. 2012;5:40-1. DOIGoogle Scholar
- Sutton-Hayes S, Weisse ME, Wilson NW, Ogershok PR. A recurrent presentation of hand, foot, and mouth disease. Clin Pediatr (Phila). 2006;45:373–6. DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers.
You must be a registered user on http://www.medscape.org. If you are not registered on http://www.medscape.org, please click on the “Register” link on the right hand side of the website.
Only one answer is correct for each question. Once you successfully answer all post-test questions, you will be able to view and/or print your certificate. For questions regarding this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@medscape.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please go to https://www.ama-assn.org. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate, and present it to your national medical association for review.
Article Title:
Epidemiology of Recurrent Hand, Foot and Mouth Disease, China, 2008–2015
CME Questions
1. Your patient is a 2-year-old Asian boy with hand-foot-and-mouth disease (HFMD). According to the surveillance study by Huang and colleagues, which of the following statements about the epidemiologic features of recurrent HFMD, using national surveillance data during 2008–2015 in mainland China, is correct?
A. Median time from primary episode to first recurrence was 24 months
B. Probability of HFMD recurrence was 1.9% at 12 months, 3.3% at 24 months, and 3.9% at 36 months, remaining stable at 4.0% at 38.8 months after the primary episode
C. The severity of HFMD was associated with probability of recurrence and with time intervals between HFMD episodes
D. Seasonal pattern was considerably different for patients with recurrent laboratory-confirmed HFMD than for those with only 1 episode of HFMD
2. According to the surveillance study by Huang and colleagues, which of the following statements about the virologic features of recurrent HFMD, using national surveillance data during 2008–2015 in mainland China, is correct?
A. Each episode of recurrent HFMD was more likely to be caused by the identical enterovirus serotype to that causing the primary episode
B. Patients with reinfection by different enterovirus serotypes differed in demographic factors from patients who were reinfected by the identical enterovirus serotype as in their primary episode
C. Of 1767 patients with laboratory-confirmed recurrence of HFMD, there were 99 with EV-A71 reinfections, 45 with CV-A16 reinfections, and 364 with other enterovirus reinfections
D. CV-A16 infections were more severe than EV-A71 infections and other EV infections
3. According to the surveillance study by Huang and colleagues, which of the following statements about the clinical implications of the epidemiologic and virologic features of recurrent HFMD, using national surveillance data during 2008–2015 in mainland China, is correct?
A. Only 1 monovalent EV-A71 vaccine has been administered in China
B. Children who receive EV-A71 vaccine are protected against HFMD
C. The burden of HFMD recurrence was likely overestimated in this study
D. To protect against an HFMD epidemic, multivalent vaccines are needed, such as EV-A71 combined with CV-A16, CV-A6, and other prevalent circulating viruses causing HFMD
Original Publication Date: February 16, 2018
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.
Related Links
Table of Contents – Volume 24, Number 3—March 2018
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Hongjie Yu, School of Public Health, Key Laboratory of Public Health Safety, Ministry of Education, Fudan University, Shanghai 200032, China; ; Sheng Wei, Department of Epidemiology and Biostatistics, Ministry of Education, Key Laboratory of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, China
Top