Sensitive and Specific Detection of Low-Level Antibody Responses in Mild Middle East Respiratory Syndrome Coronavirus Infections
Nisreen M.A. Okba, V. Stalin Raj, Ivy Widjaja, Corine H. GeurtsvanKessel, Erwin de Bruin, Felicity D. Chandler, Wan Beom Park, Nam-Joong Kim, Elmoubasher A.B.A. Farag, Mohammed Al-Hajri, Berend-Jan Bosch, Myoung-don Oh, Marion P.G. Koopmans, Chantal B.E.M. Reusken, and Bart L. Haagmans

Author affiliations: Erasmus Medical Center, Rotterdam, the Netherlands (N.M.A. Okba, V.S. Raj, C.H. GeurtsvanKessel, E. de Bruin, F.D. Chandler, M.P.G. Koopmans, C.B.E.M. Reusken, B.L. Haagmans); Utrecht University, Utrecht, the Netherlands (I. Widjaja, B.-J. Bosch); Seoul National University College of Medicine, Seoul, South Korea (W.B. Park, N.-J. Kim, M.-D. Oh); Ministry of Public Health, Doha, Qatar (E.A.B.A. Farag, M. Al-Hajri)
Main Article
Figure 1
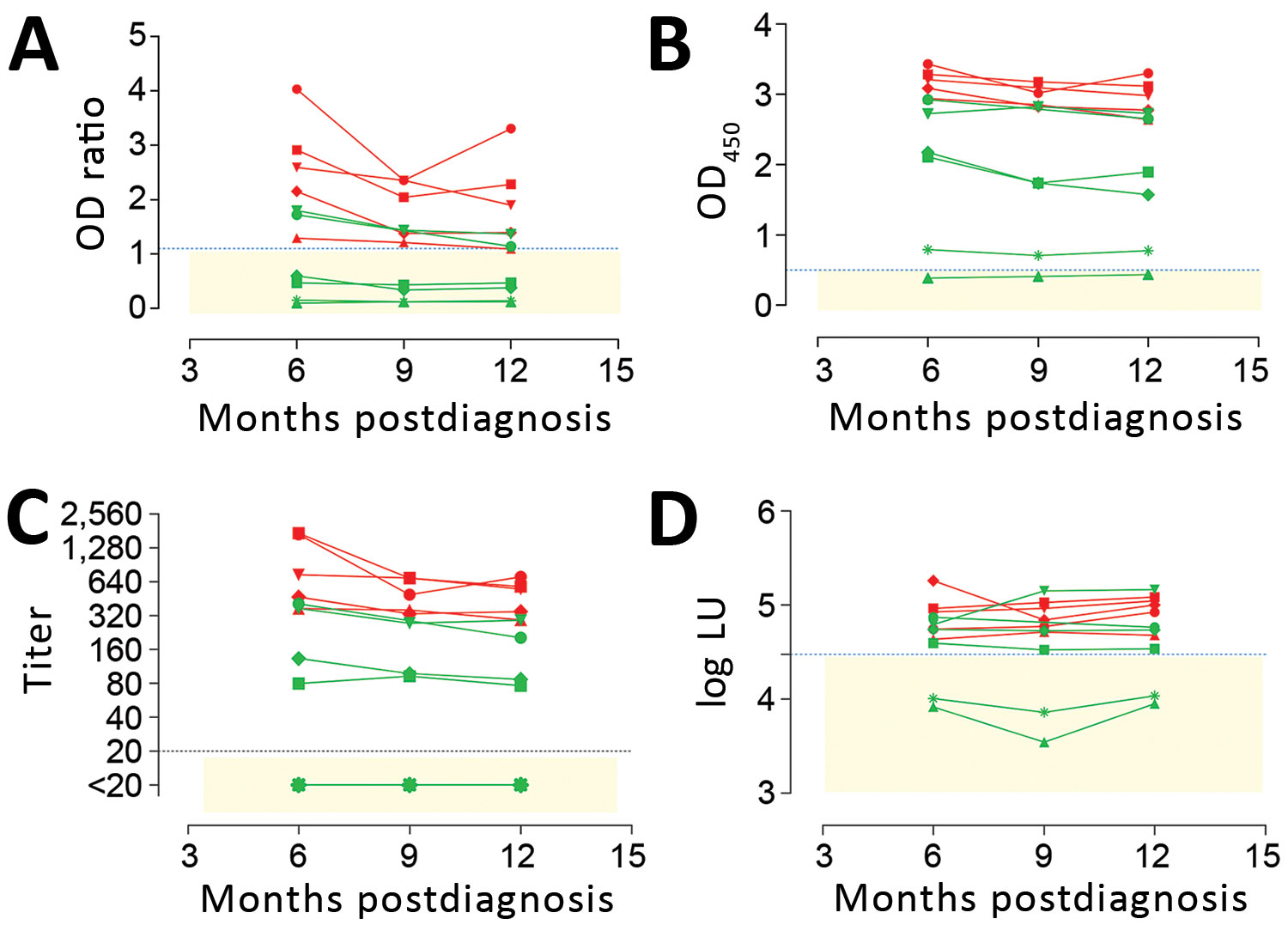
Figure 1. Detection of MERS-CoV–specific antibody responses 6–12 months following PCR-diagnosed mild and severe infections using different assays. Spike S1–specific antibody responses were tested with a routinely used S1 ELISA (rELISA) (A), in-house S1 ELISA (iELISA) (B), and S1 microarray (C). Nucleocapsid-specific antibody responses were tested using a luciferase immunoprecipitation assay (D). Severe infections (red, n = 5; cohort H) resulted in antibody responses detected for up to 1 year by all assays, while detection of mild infections (green, n = 6; cohort G) varied among assays. Horizontal dotted line indicates cutoff for each assay; yellow shaded area indicates serum undetected by each assay. CoV, coronavirus; LU, luminescence units; MERS, Middle East respiratory syndrome; OD, optical density.
Main Article
Page created: September 17, 2019
Page updated: September 17, 2019
Page reviewed: September 17, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
