Volume 25, Number 4—April 2019
Research
Differences in Neuropathogenesis of Encephalitic California Serogroup Viruses
Figure 1
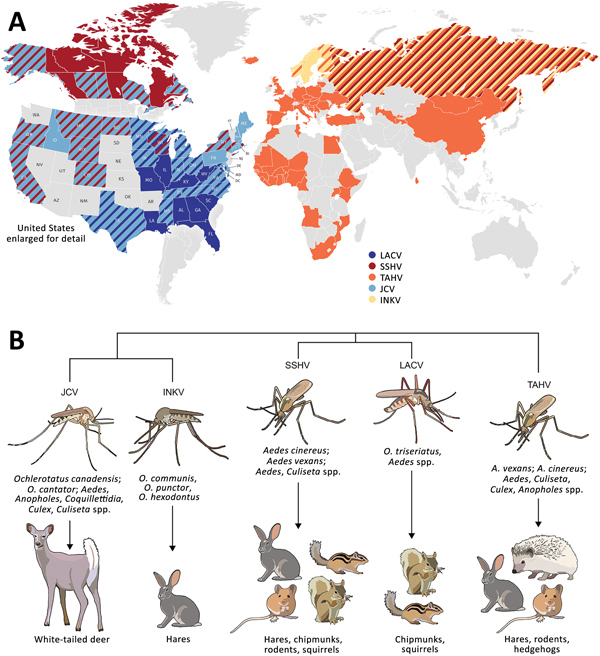
Figure 1. Global distribution, phylogenetic relationship, and vectors and hosts of the 5 California serogroup (CSG) viruses included in study of neuropathogenesis. A) These viruses are found across the globe, primarily throughout North America, Europe, Asia, and Africa (1,4,8,10,12–29). Several of these viruses have overlapping regions of distribution (as indicated by diagonal patterns), including in the United States, where LACV, SSHV, and JCV are all present, and Europe, where TAHV and INKV are present. States and countries indicated have evidence of these viruses from reported human cases, serologic surveys of humans and animals, or isolation of virus from mosquitoes. B) Within these closely related CSG viruses, JCV and INKV are the most closely related, followed by LACV and SSHV, and then TAHV (30). The CSG viruses use a variety of mosquito vectors, primarily in the Aedes and Ochlerotatus genera. Listed are the most prominent vectors and additional genera the virus has been found in. Mammals implicated as reservoir or amplifying hosts are listed for each virus; some hosts are shared among several CSG viruses (1,2,12,22,31–33). INKV, Inkoo virus; JCV, Jamestown Canyon virus; LACV, La Crosse virus; SSHV, snowshoe hare virus; TAHV, Tahyna virus.
References
- Drebot MA. Emerging mosquito-borne bunyaviruses in Canada. Can Commun Dis Rep. 2015;41:117–23. DOIPubMedGoogle Scholar
- Hubálek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008;103(Suppl 1):S29–43. DOIPubMedGoogle Scholar
- Putkuri N, Kantele A, Levanov L, Kivistö I, Brummer-Korvenkontio M, Vaheri A, et al. Acute human Inkoo and Chatanga virus infections, Finland. Emerg Infect Dis. 2016;22:810–7. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. La Crosse encephalitis. Epidemiology and geographic distribution. 2018 Jan 25 [cited 2018 May 22]. https://www.cdc.gov/lac/tech/epi.html
- Gaensbauer JT, Lindsey NP, Messacar K, Staples JE, Fischer M. Neuroinvasive arboviral disease in the United States: 2003 to 2012. Pediatrics. 2014;134:e642–50. DOIPubMedGoogle Scholar
- Lau L, Wudel B, Kadkhoda K, Keynan Y. Snowshoe hare virus causing meningoencephalitis in a young adult from northern Manitoba, Canada. Open Forum Infect Dis. 2017;4:ofx150. DOIPubMedGoogle Scholar
- Kilian P, Růzek D, Danielová V, Hypsa V, Grubhoffer L. Nucleotide variability of Tahyna virus (Bunyaviridae, Orthobunyavirus) small (S) and medium (M) genomic segments in field strains differing in biological properties. Virus Res. 2010;149:119–23. DOIPubMedGoogle Scholar
- Pastula DM, Hoang Johnson DK, White JL, Dupuis AP II, Fischer M, Staples JE. Jamestown Canyon virus disease in the United States—2000–2013. Am J Trop Med Hyg. 2015;93:384–9. DOIPubMedGoogle Scholar
- Pastula DM, Smith DE, Beckham JD, Tyler KL. Four emerging arboviral diseases in North America: Jamestown Canyon, Powassan, chikungunya, and Zika virus diseases. J Neurovirol. 2016;22:257–60. DOIPubMedGoogle Scholar
- Kosoy O, Rabe I, Geissler A, Adjemian J, Panella A, Laven J, et al. Serological survey for antibodies to mosquito-borne bunyaviruses among US National Park Service and US Forest Service employees. Vector Borne Zoonotic Dis. 2016;16:191–8. DOIPubMedGoogle Scholar
- Monath TP, Nuckolls JG, Berall , Bauer H, Chappell WA, Coleman PH. Studies on California encephalitis in Minnesota. Am J Epidemiol. 1970;92:40–50. DOIPubMedGoogle Scholar
- Putkuri N, Vaheri A, Vapalahti O. Prevalence and protein specificity of human antibodies to Inkoo virus infection. Clin Vaccine Immunol. 2007;14:1555–62. DOIPubMedGoogle Scholar
- Walters LL, Tirrell SJ, Shope RE. Seroepidemiology of California and Bunyamwera serogroup (Bunyaviridae) virus infections in native populations of Alaska. Am J Trop Med Hyg. 1999;60:806–21. DOIPubMedGoogle Scholar
- Haddow AD, Odoi A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003-2007. PLoS One. 2009;4:e6145. DOIPubMedGoogle Scholar
- Clark GG, Crabbs CL, Watts DM, Bailey CL. An ecological study of Jamestown Canyon virus on the Delmarva Peninsula, with emphasis on its possible vector. J Med Entomol. 1986;23:588–99. DOIPubMedGoogle Scholar
- Boromisa RD, Grimstad PR. Virus-vector-host relationships of Aedes stimulans and Jamestown Canyon virus in a northern Indiana enzootic focus. Am J Trop Med Hyg. 1986;35:1285–95. DOIPubMedGoogle Scholar
- Pinger RR, Rowley WA, Wong YW, Dorsey DC. Trivittatus virus infections in wild mammals and sentinel rabbits in central Iowa. Am J Trop Med Hyg. 1975;24:1006–9. DOIPubMedGoogle Scholar
- Heard PB, Zhang MB, Grimstad PR. Isolation of Jamestown Canyon virus (California serogroup) from Aedes mosquitoes in an enzootic focus in Michigan. J Am Mosq Control Assoc. 1990;6:461–8.PubMedGoogle Scholar
- Anderson JF, Main AJ, Armstrong PM, Andreadis TG, Ferrandino FJ. Arboviruses in North Dakota, 2003-2006. Am J Trop Med Hyg. 2015;92:377–93. DOIPubMedGoogle Scholar
- McFarlane BL, Embil JA, Artsob H, Spence L, Rozee KR. Antibodies to the California group of arboviruses in the moose (Alces alces americana Clinton) population of Nova Scotia. Can J Microbiol. 1981;27:1219–23. DOIPubMedGoogle Scholar
- Berry RL, Weigert BJL, Calisher CH, Parsons MA, Bear GT. Evidence for transovarial transmission of Jamestown Canyon virus in Ohio. Mosq News. 1977;37:494–6.
- Issel CJ, Hoff GL, Trainer DO. Serologic evidence of infection of white-tailed deer in Texas with three California group arboviruses, (Jamestown Canyon, San Angelo, and Keystone). J Wildl Dis. 1973;9:245–8. DOIPubMedGoogle Scholar
- Tyers D, Zimmer J, Lewandowski K, Hennager S, Young J, Pappert R, et al. Serologic survey of snowshoe hares (Lepus americanus) in the Greater Yellowstone Area for brucellosis, tularemia, and snowshoe hare virus. J Wildl Dis. 2015;51:769–73. DOIPubMedGoogle Scholar
- Campbell GL, Eldridge BF, Hardy JL, Reeves WC, Jessup DA, Presser SB. Prevalence of neutralizing antibodies against California and Bunyamwera serogroup viruses in deer from mountainous areas of California. Am J Trop Med Hyg. 1989;40:428–37. DOIPubMedGoogle Scholar
- Eldridge BF, Calisher CH, Fryer JL, Bright L, Hobbs DJ. Serological evidence of California serogroup virus activity in Oregon. J Wildl Dis. 1987;23:199–204. DOIPubMedGoogle Scholar
- Issel CJ, Trainer DO, Thompson WH. Serologic evidence of infections of white-tailed deer in Wisconsin with three California group arboviruses (La Crosse, trivittatus, and Jamestown Canyon). Am J Trop Med Hyg. 1972;21:985–8. DOIPubMedGoogle Scholar
- Srihongse S, Grayson MA, Deibel R. California serogroup viruses in New York State: the role of subtypes in human infections. Am J Trop Med Hyg. 1984;33:1218–27. DOIPubMedGoogle Scholar
- Walker ED, Grayson MA, Edman JD. Isolation of Jamestown Canyon and snowshoe hare viruses (California serogroup) from Aedes mosquitoes in western Massachusetts. J Am Mosq Control Assoc. 1993;9:131–4.PubMedGoogle Scholar
- Jentes ES, Robinson J, Johnson BW, Conde I, Sakouvougui Y, Iverson J, et al. Acute arboviral infections in Guinea, West Africa, 2006. Am J Trop Med Hyg. 2010;83:388–94. DOIPubMedGoogle Scholar
- Hughes HR, Lanciotti RS, Blair CD, Lambert AJ. Full genomic characterization of California serogroup viruses, genus Orthobunyavirus, family Peribunyaviridae including phylogenetic relationships. Virology. 2017;512:201–10. DOIPubMedGoogle Scholar
- Pantuwatana S, Thompson WH, Watts DM, Hanson RP. Experimental infection of chipmunks and squirrels with La Crosse and Trivittatus viruses and biological transmission of La Crosse virus by Aedes triseriatus. Am J Trop Med Hyg. 1972;21:476–81. DOIPubMedGoogle Scholar
- Artsob H. Distribution of California serogroup viruses and virus infections in Canada. Prog Clin Biol Res. 1983;123:277–90.PubMedGoogle Scholar
- Andreadis TG, Anderson JF, Armstrong PM, Main AJ. Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: a ten-year analysis, 1997-2006. Vector Borne Zoonotic Dis. 2008;8:175–88. DOIPubMedGoogle Scholar
- Kunz C, Buckley SM, Casals J. Antibodies in man against Tahyna and Lumbo viruses determined by hemagglutination-inhibition and tissue-culture neutralization tests. Am J Trop Med Hyg. 1964;13:738–41. DOIPubMedGoogle Scholar
- Sonnleitner ST, Lundström J, Baumgartner R, Simeoni J, Schennach H, Zelger R, et al. Investigations on California serogroup orthobunyaviruses in the Tyrols: first description of Tahyna virus in the Alps. Vector Borne Zoonotic Dis. 2014;14:272–7. DOIPubMedGoogle Scholar
- Kuniholm MH, Wolfe ND, Huang CYH, Mpoudi-Ngole E, Tamoufe U, LeBreton M, et al. Seroprevalence and distribution of Flaviviridae, Togaviridae, and Bunyaviridae arboviral infections in rural Cameroonian adults. Am J Trop Med Hyg. 2006;74:1078–83. DOIPubMedGoogle Scholar
- Elliott RM. Orthobunyaviruses: recent genetic and structural insights. Nat Rev Microbiol. 2014;12:673–85. DOIPubMedGoogle Scholar
- Blakqori G, Delhaye S, Habjan M, Blair CD, Sánchez-Vargas I, Olson KE, et al. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J Virol. 2007;81:4991–9. DOIPubMedGoogle Scholar
- Taylor KG, Woods TA, Winkler CW, Carmody AB, Peterson KE. Age-dependent myeloid dendritic cell responses mediate resistance to la crosse virus-induced neurological disease. J Virol. 2014;88:11070–9. DOIPubMedGoogle Scholar
- Bennett RS, Gresko AK, Murphy BR, Whitehead SS. Tahyna virus genetics, infectivity, and immunogenicity in mice and monkeys. Virol J. 2011;8:135. DOIPubMedGoogle Scholar
- Bennett RS, Nelson JT, Gresko AK, Murphy BR, Whitehead SS. The full genome sequence of three strains of Jamestown Canyon virus and their pathogenesis in mice or monkeys. Virol J. 2011;8:136. DOIPubMedGoogle Scholar
- Butchi NB, Woods T, Du M, Morgan TW, Peterson KE. TLR7 and TLR9 trigger distinct neuroinflammatory responses in the CNS. Am J Pathol. 2011;179:783–94. DOIPubMedGoogle Scholar
- Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, et al. Adaptive immune responses to Zika virus are important for controlling virus infection and preventing infection in brain and testes. J Immunol. 2017;198:3526–35. DOIPubMedGoogle Scholar
- Bennett RS, Cress CM, Ward JM, Firestone CY, Murphy BR, Whitehead SS. La Crosse virus infectivity, pathogenesis, and immunogenicity in mice and monkeys. Virol J. 2008;5:25. DOIPubMedGoogle Scholar
- Winkler CW, Race B, Phillips K, Peterson KE. Capillaries in the olfactory bulb but not the cortex are highly susceptible to virus-induced vascular leak and promote viral neuroinvasion. Acta Neuropathol. 2015;130:233–45. DOIPubMedGoogle Scholar
- Winkler CW, Myers LM, Woods TA, Carmody AB, Taylor KG, Peterson KE. Lymphocytes have a role in protection, but not in pathogenesis, during La Crosse Virus infection in mice. J Neuroinflammation. 2017;14:62. DOIPubMedGoogle Scholar