Volume 27, Number 1—January 2021
Research
Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2–Specific IgG
Figure 2
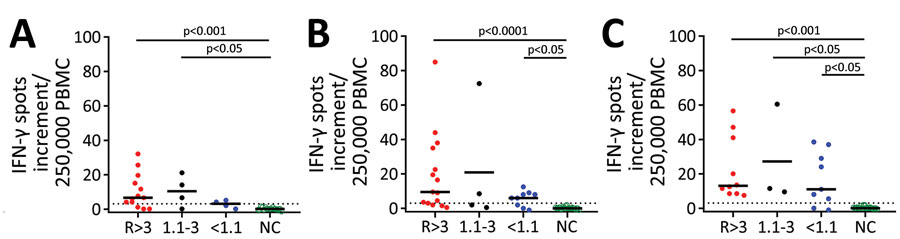
Figure 2. Cellular immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as determined by ELISpot assay in potential convalescent-plasma donors with PCR-confirmed infection, Germany. Peripheral blood mononuclear cells of volunteers were stimulated by an S1 protein antigen of SARS-CoV-2 (A) and by peptide pools of the S1/S2 (B) and the M protein (C). If volunteers were tested sequentially, we only included the first dataset. The 3 left groups represent potential convalescent-plasma donors with PCR-confirmed SARS-CoV-2 infection. They either had a strong positive antibody response to the SARS-CoV-2 IgG ELISA as defined by an antibody ratio (R) of >3 (n = 15), an intermediate response (ratio of 1.1–3, n = 4) or borderline or negative results (ratio of <1.1, n = 9). The right group displays data in healthy controls without symptoms of respiratory or gastrointestinal infections and without household contact with SARS-CoV-2 infected patients since January 2020 (n = 22). The group has tested negative or has not been tested by SARS-CoV-2 PCR. Responses in the 4 groups of volunteers were compared by Kruskal-Wallis test with Dunn’s correction. Dotted lines represent 3 spots increment. Horizontal lines indicate median values. Stimulation by S1 protein could not be performed in 7 volunteers; stimulation by the M peptide pool could not be performed in 6. Red dots represent volunteers with an antibody ratio >3; black dots, volunteers with a ratio of 1.1–3; blue dots, volunteers with ratio <1.1; green, NC. IFN-γ, interferon-γ; NC, negative controls.