Volume 27, Number 2—February 2021
Research Letter
Severe Acute Respiratory Syndrome Coronavirus 2 Outbreak Related to a Nightclub, Germany, 2020
Cite This Article
Citation for Media
Abstract
We report an outbreak of coronavirus disease with 74 cases related to a nightclub in Germany in March 2020. Staff members were particularly affected (attack rate 56%) and likely caused sustained viral transmission after an event at the club. This outbreak illustrates the potential for superspreader events and corroborates current club closures.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) superspreading events are particularly linked to indoor settings, such as religious venues (1), restaurants (2), and bars or nightclubs (3–6). To provide further details on the extent and transmission dynamics in nightclubs, we describe a SARS-CoV-2 outbreak related to a Berlin, Germany, nightclub during the early phase of the coronavirus disease (COVID-19) pandemic, before infection prevention measures were applied.
On March 5, 2020, contact tracing activities in Berlin revealed several COVID-19 cases linked by visiting the same nightclub, club X, on February 29, 2020 (event 1). Estimates suggest ≈300 guests attended event 1. Club X then held other events: event 2 with ≈150 guests on March 2 and event 3 with ≈200 guests on March 5. On March 6, the local health authority of Mitte district, Berlin, published announcements in local newspapers and on social media to identify other attendees of the events. Everyone attending >1 event was categorized as a high-risk contact person and ordered to self-quarantine for 14 days. If symptoms occurred, laboratory testing was recommended. Mandatory case notification occurred from the laboratory to the local health authority based on Germany’s Protection against Infection Act (7). Due to the increasing spread of COVID-19, on March 16, 2020, government authorities in Germany prohibited social gatherings, including events in nightclubs, until further notice.
Confirmed cases in the outbreak were defined as persons with laboratory-confirmed SARS-CoV-2 (Appendix). We retrieved dates of symptom onset and sociodemographic data of 64 outbreak cases from the national infectious diseases notification database. We considered staff and persons who attended any event at club X to have first-generation cases and their contacts to have second-generation cases.
We interviewed 44 persons with first-generation cases whose contact information was available and with all 16 club X staff members who worked any of the 3 events. For staff members who were not tested after the events or who tested negative despite reporting symptoms, we offered SARS-CoV-2 antibody testing 3 months after the outbreak to ascertain their infection status. We also mapped the space inside club X (Appendix Figure 1).
In total, 74 reported cases were linked to the outbreak. Median age was 30 (range 2–63) years; cases were equally distributed by sex, 37 female (50%) and 37 male (50%). Among 41 first-generation cases with known date of symptom onset and only 1 exposure, the median incubation period was 4 days (interquartile range 3–6 days). The calculated attack rates (ARs) show that guests attending event 1 were particularly affected. Staff pooled over all events had the highest risk for infection (AR 56%) (Table).
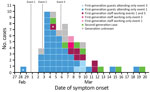
Among guests, 1 PCR-confirmed case had self-reported initial symptoms 1 day before attending event 1 and could be a potential source of the outbreak. The most probable source for continued viral transmission at event 3 was a PCR-confirmed case in a staff member working event 1 and event 3, with symptom onset 1 day before event 3. Overall, staff members reported symptom onset at a later stage of the outbreak than guests (Figure).
SARS-CoV-2 whole-genome sequencing was performed on 17 available patient samples to assess clustering of sequences. Sequencing revealed that 10 cases among event 1 guests, 2 second-generation cases, and 5 cases of unknown generation all grouped within clade G (GISAID, https://www.gisaid.org) and B.1 (Pangolin clade naming) (Appendix Figure 2). This clade also was observed in the SARS-CoV-2 outbreak in Italy and many later outbreaks in Europe (8). Sequences from 11 samples were identical. The other 6 samples were otherwise identical, but had slight differences; 1 sequence had 1 position with ambiguous nucleotides; 3 other sequences had 3 positions with ambiguous nucleotides; 1 sequence had a substitution in the 3′ untranslated region; and sequences from 2 cases, in a couple who attended event 1, had an identical substitution in the N gene (Appendix Table 1). This substitution could hint to a second independent transmission cluster comprising these 2 cases, but all observed sequence variants also can be explained by sporadic mutation events. Thus, the sequence data do not provide evidence against a single person as the outbreak source (Appendix Figure 2).
The large number of cases from event 1, the relatively low median incubation period (4 days) for first-generation cases, and the close genetic relatedness of the sequenced viruses corroborate the theory of transmission from a single person and the potential for superspreading in a nightclub when no social distancing measures are applied. This outbreak further illustrates the potential role of nightclub staff members in transmission. AR among staff was particularly high (56%), showing they had a particularly high risk for infection. Because 1 staff member appears to have been infected at event 1, then worked with symptoms at event 3, continued viral transmission could have been caused by staff. However, without sequencing data for all cases, staff contribution to viral transmission cannot be confirmed. Nonetheless, once ease of restrictions is considered, our study suggests that infection protection should be targeted particularly toward staff in nightclubs and bars.
Dr. Muller is a medical doctor working as a fellow of the Postgraduate Training for Applied Epidemiology at the Robert Koch Institute, Berlin, and has a research affiliation with the Department of Infectious Diseases and Respiratory Medicine, Charité University Medicine Berlin. Her main research interests include epidemiological investigations on respiratory diseases.
Acknowledgments
We thank all our colleagues in the local health authority in Berlin and other federal states who actively collaborated in case finding activities, especially the team from the local health authority in the Berlin district Mitte for their unremitting commitment in managing cases and contact persons during the outbreak. We also thank the affected nightclub’s owner and staff members for their trustful sharing of information. This study was only possible thanks to the collaboration of the affected individuals from the outbreak. We also gratefully acknowledge the authors, originating and submitting laboratories of the genetic sequence and metadata made available through GISAID on which the phylogenetic analysis presented in this paper is based.
N.M. is a fellow of Postgraduate Training for Applied Epidemiology, supported financially by the Robert Koch Institute. N.J.S. is a fellow of the European Centre for Disease Prevention and Control (ECDC) Fellowship Programme, supported financially by the ECDC. Contributions by V.M.C. and C.D. were funded by the German Ministry of Health (Konsiliarlabor für Coronaviren) and the German Center for Infection Research (DZIF).
References
- Leclerc QJ, Fuller NM, Knight LE, Funk S, Knight GM; CMMID COVID-19 Working Group. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. DOIPubMedGoogle Scholar
- Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628–31. DOIPubMedGoogle Scholar
- Abbott S, Hellewell J, Thompson RN, Sherratt K, Gibbs HP, Bosse NI, et al. Estimating the time-varying reproduction number of SARS-CoV-2 using national and subnational case counts. Wellcome Open Res. 2020;5:112. DOIGoogle Scholar
- Correa-Martínez CL, Kampmeier S, Kümpers P, Schwierzeck V, Hennies M, Hafezi W, et al. A pandemic in times of global tourism: superspreading and exportation of COVID-19 cases from a ski area in Austria. J Clin Microbiol. 2020;58:3. DOIPubMedGoogle Scholar
- Kang CR, Lee JY, Park Y, Huh IS, Ham HJ, Han JK, et al.; Seoul Metropolitan Government COVID-19 Rapid Response Team (SCoRR Team). (SCoRR Team). Coronavirus disease exposure and spread from nightclubs, South Korea. Emerg Infect Dis. 2020;26:2499–501. DOIPubMedGoogle Scholar
- Chau NVV, Hong NTT, Ngoc NM, Thanh TT, Khanh PNQ, Nguyet LA, et al. Superspreading event of SARS-CoV-2 infection at a bar, Ho Chi Minh City, Vietnam. Emerg Infect Dis. 2020 Oct 16 [Epub ahead of print].
- Federal Ministry for Justice and Consumer Protection. Law on the Prevention and Control of Infectious Diseases in Humans [in German] [cited 2020 Nov 27]. https://www.gesetze-im-internet.de/ifsg/index.html
- Rambaut A, Holmes EC, O’Toole Á, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–7. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: December 02, 2020
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.
Table of Contents – Volume 27, Number 2—February 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nadine Muller, Department of Infectious Disease Epidemiology, Robert Koch Institute, Seestrasse 10, Berlin 13353, Germany
Top