Volume 28, Number 4—April 2022
Research Letter
Autochthonous Leishmania infantum in Dogs, Zambia, 2021
Cite This Article
Citation for Media
Abstract
Leishmaniases are neglected tropical diseases of humans and animals. We detected Leishmania infantum in 3 mixed-breed dogs in Zambia that had no travel history outside the country. Our findings suggest presence of and probable emergence of leishmaniasis in Zambia, indicating the need for physicians and veterinarians to consider the disease during diagnosis.
Leishmaniasis, a neglected tropical disease of humans and animals, is estimated to affect <1 million persons annually (https://www.who.int/health-topics/leishmania). The disease is caused by intracellular protozoan parasites of the genus Leishmania (Trypanosomatida: Trypanosomatidae), which are vectored by female sand flies of the genera Phlebotomus in the Old World and Lutzomyia in the New World (1). Although leishmaniasis is endemic to several resource-poor countries in eastern, western, and northern Africa, there is a dearth of information on the epidemiology of the disease in southern Africa, largely caused by weak surveillance systems (1,2).
In Zambia, human visceral leishmaniasis was reported in 1973 in the Eastern Province (3) and subsequently in 1976 in the same area (4). In 1994, a case of canine visceral leishmaniasis was reported in a dog in Lusaka Province (5). We report detection of canine leishmaniasis caused by Leishmania infantum, suggesting possible reemergence or reintroduction of the disease in Zambia.
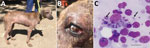
The study was approved by the Department of Veterinary Services, Government of the Republic of Zambia. In June 2021, two female mixed-breed shelter dogs (case 1 and case 2) rescued in Southern Province of Zambia during 2020 were brought to a veterinary clinic in Lusaka. These 2 dogs had chronic weight loss, generalized alopecia, and ulcerative and exfoliative dermatitis (Figure, panel A). The dogs had been previously treated for tickborne and helminth infections but showed no improvement. Physical examination showed that the prescapular and popliteal lymph nodes were enlarged. Case 1 had onychogryphosis (Figure, panel A) and focal corneal opacity in the left eye (Figure, panel B).
Biochemistry profiles for both dogs showed increased levels of serum proteins (>93.3 g/L) and hyperglobulinemia (>74.9 g/L) and hypoalbuminemia (<14.7 g/L), which are common manifestations suggestive of canine leishmaniasis. Giemsa staining of fine-needle lymph node aspirate identified Leishmania spp. amastigotes (Figure, panel C).
We performed serologic analysis of Leishmania antibodies by using L. donovani soluble lysate antigen from cultured promastigotes and recombinant LinJ14.1160r4 antigens as described (6). Both dogs had high antibody titers, >1.0 optical density units (Appendix Figure). Among in-contact dogs from Southern Province that had no clinical signs (n = 6), 1 dog (case 3) had high antibody titers for both assays, and 2 dogs (dogs 6 and 7) had high antibody titers for LinJ14.1160r4 only. All control serum samples (n = 39) from Central Province were negative for Leishmania antibodies on both assays (Appendix Figure). For the purpose of disease control and the absence of antileishmanial agents in Zambia, the dogs (cases 1 and 2) were euthanized by rapid intravenous infusion of pentobarbitone sodium (0.7 mL/kg body weight). Case 3, an in-contact dog that showed high antibody titers for both assays, was euthanized 1 month after clinical disease was detected and the initial diagnosis.
At necropsy, we aseptically harvested spleen tissue and processed this tissue for genomic DNA extraction by using the QuickGene DNA Tissue Kit (Kurabo, https://www.kurabo.co.jp) according to the manufacturer’s protocol. We performed PCRs targeting the partial small subunit ribosomal RNA gene (7) and internal transcribed spacer (ITS) 1 and ITS 2 genes (8). PCR for 3 dogs showed expected band sizes, which we purified and sequenced on a 3500 Genetic Analyzer (Applied Biosystems, https://www.thermofisher.com). Sequences obtained were 100% identical with the L. infantum reference strain (JPCM5) isolated in Spain (9). The ITS sequence type was type A, which was assigned according to 12 microsatellite regions in ITS1 and ITS2 within the L. donovani complex (8). ITS type A is the dominant L. infantum type reported mainly from the Mediterranean basin, and types D, E, F, and G are associated with L. donovani from eastern Africa (8). Nucleotide sequences from this study were deposited in the DNA DataBank of Japan (GenBank accession nos. LC652643‒LC652645).
Our study confirmed the presence and probable emergence of leishmaniasis and Leishmania parasites in Zambia. An in-contact, seropositive dog that did not have clinical signs had clinical disease develop 1 month after the initial diagnosis. However, the probable route of infection remains unclarified.
Although the geographic distribution of vector sandflies has not been described in Zambia, neighboring countries have reported presence of Phlebotomus spp. sand flies (10). In addition, although the extent of disease distribution in the country, including Southern Province, is yet to be determined, autochthonous leishmania cases reported in Zambia (3–5) suggests the presence of an infection foci. To further clarify the epidemiology of leishmaniasis in Zambia, there is need for improved understanding of the epidemiology of the disease in dogs, vector distribution, and the risk for human infection, particularly in high-risk populations, such as immunocompromised persons.
Dr. Squarre is a state wildlife veterinarian in Lusaka, Zambia. His research interests include molecular epidemiology of emerging and reemerging zoonosis in free-ranging wildlife and domestic animals and their interaction at the human‒wildlife‒livestock interface.
Acknowledgment
This study was supported in part by the Department of Veterinary Services under the Ministry of Fisheries and Livestock of the Government of the Republic of Zambia, the Japan Agency for Medical Research and Development (JP21wm0125008), and the Lusaka Animal Welfare Society.
References
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:
e35671 . DOIPubMedGoogle Scholar - Otranto D, Dantas-Torres F, Mihalca AD, Traub RJ, Lappin M, Baneth G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017;33:813–25. DOIPubMedGoogle Scholar
- Hira PR, Naik KG, Egere JU. Letter: Kala-azar in Zambia. Lancet. 1973;302:1026–7. DOIPubMedGoogle Scholar
- Naik KG, Hira PR, Bhagwandeen SB, Egere JU, Versey AA. Kala-azar in Zambia: first report of two cases. Trans R Soc Trop Med Hyg. 1976;70:328–32. DOIPubMedGoogle Scholar
- Matsukawa K, Chiti L, Yoshima M, Sayer PD. Canine visceral leishmaniosis: first case in Zambia. Onderstepoort J Vet Res. 1997;64:77–9.PubMedGoogle Scholar
- Goto Y, Carter D, Guderian J, Inoue N, Kawazu S, Reed SG. Upregulated expression of B-cell antigen family tandem repeat proteins by Leishmania amastigotes. Infect Immun. 2010;78:2138–45. DOIPubMedGoogle Scholar
- Meredith SE, Zijlstra EE, Schoone GJ, Kroon CC, van Eys GJ, Schaeffer KU, et al. Development and application of the polymerase chain reaction for the detection and identification of Leishmania parasites in clinical material. Arch Inst Pasteur Tunis. 1993;70:419–31.PubMedGoogle Scholar
- el Tai NO, Osman OF, el Fari M, Presber W, Schönian G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 2000;94:575–9. DOIPubMedGoogle Scholar
- González-de la Fuente S, Peiró-Pastor R, Rastrojo A, Moreno J, Carrasco-Ramiro F, Requena JM, et al. Resequencing of the Leishmania infantum (strain JPCM5) genome and de novo assembly into 36 contigs. Sci Rep. 2017;7:18050. DOIPubMedGoogle Scholar
- Seccombe AK, Ready PD, Huddleston LM. A catalogue of Old World phlebotomine sandflies (Diptera: Psychodidae, Phlebotominae). (Occasional papers on systematic entomology, number 8.) Portsmouth (UK): Natural History Museum; 1993.
Figure
Cite This ArticleOriginal Publication Date: March 03, 2022
1These authors contributed equally to this article
Table of Contents – Volume 28, Number 4—April 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Herman M. Chambaro, Ministry of Fisheries and Livestock, Department of Veterinary Services, Central Veterinary Research Institute, PO Box 33980, Lusaka, Zambia
Top