Volume 28, Number 7—July 2022
Research
One Health Genomic Analysis of Extended-Spectrum β-Lactamase‒Producing Salmonella enterica, Canada, 2012‒2016
Cite This Article
Citation for Media
Abstract
Extended-spectrum β-lactamases (ESBLs) confer resistance to extended-spectrum cephalosporins, a major class of clinical antimicrobial drugs. We used genomic analysis to investigate whether domestic food animals, retail meat, and pets were reservoirs of ESBL-producing Salmonella for human infection in Canada. Of 30,303 Salmonella isolates tested during 2012–2016, we detected 95 ESBL producers. ESBL serotypes and alleles were mostly different between humans (n = 54) and animals/meat (n = 41). Two exceptions were blaSHV-2 and blaCTX-M-1 IncI1 plasmids, which were found in both sources. A subclade of S. enterica serovar Heidelberg isolates carrying the same IncI1-blaSHV-2 plasmid differed by only 1–7 single nucleotide variants. The most common ESBL producer in humans was Salmonella Infantis carrying blaCTX-M-65, which has since emerged in poultry in other countries. There were few instances of similar isolates and plasmids, suggesting that domestic animals and retail meat might have been minor reservoirs of ESBL-producing Salmonella for human infection.
Nontyphoidal Salmonella is a leading cause of foodborne diarrheal illness. There were an estimated 535,000 human cases of invasive infection with nontyphoidal Salmonella and 59,100 deaths in 2017 globally (1,2). Gastroenteritis is usually self-limiting, but antimicrobial treatment, including ceftriaxone, ciprofloxacin, trimethoprim/sulfamethoxazole, or amoxicillin, might be recommended for severe disease and invasive infections (3).
Extended-spectrum cephalosporins are a major class of broad-spectrum antimicrobial drugs and can be hydrolyzed by β-lactamases belonging to molecular class C (AmpC type, such as blaCMY-2) and molecular class A (ESBLs, such as blaCTX-M, blaSHV, and some alleles of blaTEM) (4). ESBLs are a special concern because they sometimes cause reduced susceptibility to fourth-generation cephalosporins, such as cefepime, and they tend to be carried on mobile genetic elements (5). ESBLs are susceptible to β-lactamase inhibitors (e.g., clavulanic acid) and cephamycin-type cephalosporins (e.g., cefoxitin). Potential reservoirs of antimicrobial drug resistance are the food chain, the community, hospitals, and the environment (6). Ceftiofur was used systematically in the poultry industry in Canada. However, as of 2014, the industry voluntarily eliminated preventive use of antimicrobial drugs that were highly essential, including ceftiofur (7). As of December 1, 2018, medically essential antimicrobial drugs are available only by veterinary prescription for use in animals in Canada (8).
The most common ESBL-producing Enterobacterales are Escherichia coli and Klebsiella pneumoniae carrying CTX-M enzymes, but ESBL Salmonella are observed infrequently (9–11). Approximately 10% of human clinical isolates of E. coli and K. pneumoniae were ESBL producing in Canada during 2016 (9). Three previous studies of a combined total of >11,000 Salmonella isolates from humans and animals/meat in North America identified only 7 ESBL isolates during 2005–2008 (12–14).
We conducted a genomic study of surveillance isolates collected by the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) to evaluate the contribution of food animals, retail meat, and pets toward human infections of ESBL-producing Salmonella in Canada during 2012–2016. We also characterized ESBL plasmids by short-read and long-read whole-genome sequencing (WGS).
ESBL Detection
ESBL-producing typhoidal and nontyphoidal Salmonella collected from 2012–2016 were identified by CIPARS, which collects human clinical samples from all 10 provincial public health laboratories in Canada. CIPARS also collects animals/meat isolates from farms, abattoirs, and retail stores, and veterinary diagnostic samples from animal health laboratories (15). We conducted antimicrobial drug susceptibility testing by using broth microdilution, the Sensititer Automated Microbiology System (Trek Diagnostic Systems Ltd., https://www.trekds.com), and breakpoints established by the Clinical Laboratory Standards Institute (16). CIPARS carries out susceptibility testing on all Salmonella serotypes from animals/meat sources and 11 serotypes from human samples that are either frequently isolated or frequently multidrug-resistant (4,[5],12,i:-, Dublin, Enteritidis, Heidelberg, Infantis, Kentucky, Newport, Paratyphi A, Paratyphi B, Typhi, and Typhimurium). We performed the combination ESBL disk test (cefotaxime or ceftazidime alone or in combination with clavulanic acid) on human isolates belonging to serotypes that were identified as ESBL producing in animals/meat that were not initially tested by broth microdilution (Anatum, Worthington Agona, Albany, Bredeney, Brandenburg, California, Derby, Ohio, and Ouakam).
We subjected isolates that had a ceftriaxone MIC >0.5 mg/L to the ESBL disk test and a β-lactam PCR to detect blaCMY-2, blaTEM, blaCTX-M, blaSHV, and blaOXA as described (17). Isolates were selected for WGS if they showed positive results in the ESBL disk test or if they contained blaCTX-M, blaSHV, or blaOXA. To capture ESBL variants of blaTEM, we also sequenced isolates that were positive for blaTEM by PCR but lacked CMY-2 or another ESBL-hydrolyzing enzyme.
Short-Read WGS
We subjected potential ESBL-producing Salmonella to short-read sequencing. We extracted DNA by using the Epicenter Complete DNA and RNA Extraction Kit (Illumina, https://www.illumina.com). We prepared libraries by using the Nextera XT Kit and sequenced them on the Miseq Platform with the Miseq Reagent v3 600 Cycle Kit (both from Illumina) The average genome coverage was 95× (range 68×‒147×), and the average N50 (length of the shortest contig in the group of longest contigs that together represent >50% of genome assembly) of assemblies was 411,319 bp (range 79,379‒740,528 bp), indicating high quality of sequencing and assemblies.
Long-Read WGS
We conducted long-read WGS on a subset of isolates by using the MinION Platform (Oxford Nanopore Technologies, https://nanoporetech.com). We prepared libraries by using the Rapid Barcoding SQK-RBK004 Kit and sequenced them by using R9.4 Flowcells (both from Oxford Nanopore Technologies). All blaSHV-2 isolates were selected for long-read sequencing because this gene was commonly detected in humans (n = 12) and animals/meat (n = 15). If an ESBL variant was observed >5 times in 1 source (humans or animals/meat), we selected a convenience sample of 3 isolates from that source for long-read sequencing (blaCTX-M-55 and blaCTX-M-65 in humans and blaCTX-M-1 in animals/meat). If the ESBL enzyme was observed <5 times in 1 source, we selected 1 isolate for long-read sequencing (blaSHV-2, blaCTX-M-1, blaCTX-M-3, blaCTX-M-9, blaCTX-M-14, and blaCTX-M-15 in humans and blaCTX-M-55 in animals/meat).
Assembly and Alignment
We assembled short reads by using SPAdes Galaxy version 3.11.1 (18). We conducted plasmid assembly by using Unicycler version 0.4.7, which combines the accuracy of short reads with the scaffolding of long reads (19). Determinants of antimicrobial drug resistance and plasmids were detected by using the Public Health Agency of Canada StarAMR Tool (20), which incorporates the ResFinder, PointFinder, and PlasmidFinder databases (21,22). We created plasmid alignments by using the web-based GView server (https://www.server.gview.ca) parameters: minimum length 150 and minimum nucleotide identity 98%. If an alignment included only plasmids that were closed by long-read sequencing, we used the pangenome feature of GView, which displays all content for all plasmids. If an alignment included any samples that were subjected only to short-read sequencing (fragmented assemblies), we used the BLAST atlas feature of GView, which displays only homology to a closed reference plasmid. We described plasmids as being similar if they displayed >95% nucleotide identity over >90% of the length of the plasmid.
Phylogenetic Trees
We used the single-nucleotide variant (SNV) phylogenomics (SNVPhyl) pipeline (https://snvphyl.readthedocs.io/en/latest) to build genomic dendrograms based on SNVs in the core genome (23). In brief, we mapped reads to a reference genome by using SMALT (https://bio.tools/smalt). We called variants by using mpileup (http://comailab.genomecenter.ucdavis.edu/index.php/mpileup) and Freebayes (https://www.geneious.com/plugins/freebayes), and used consensus SNVs to build the dendrogram by using PhyML (24) and the generalized time reversible model. Parameters used were minimum coverage 10, minimum mapping quality 30, and SNV density filtering > 2 SNVs/20 base window. SH-like branch support values >0.95 were considered to be strong support for internal branches (24).
Accession Identifiers
We deposited read data for all isolates in this study to the National Center for Biotechnology Information Short Read Archive under BioProject PRJNA740259. We provided the BioSample identification for the isolates (Appendix Table).
WGS of ESBL-Producing Salmonella
During 2012–2016, the prevalence of class A ESBL enzymes in Canada was 0.35% in humans (54 ESBL-positive/15,401 screened) and 0.31% in the animals/meat and pet samples (41 ESBL-positive/14,923 screened) within CIPARS. The animals/meat-source Salmonella were from turkey/turkeys (refers to meat/animal; 5 from meat, 17 from animals), pigs (n = 11, all animals), cattle (n = 4, all animals), chicken/chickens (1 from meat, 2 from animals), and domestic cat (n = 1) (Table 1). Thus, only 6 samples were from meat, and the remaining 36 samples were from animals, including healthy animals on farms (n = 11, 26.8%), and veterinary clinical samples from sick animals (n = 24, 68.5%). The human-source Salmonella were from stool (n = 48), blood (n = 2), urine (n = 2), and unknown sources (n = 2). We provided detailed information on all isolates (Appendix Table).
ESBL Serotypes and Alleles
ESBLs were detected in a variety of Salmonella serotypes from human sources (54 isolates belonging to 11 serotypes) (Table 2) and animals/meat sources (41 isolates belonging to 14 serotypes) (Table 3). In humans, the most common ESBL-producing serotypes were Heidelberg (n = 16; 29.6%), Infantis (n = 15; 27.8%), Typhimurium (n = 7; 13.0%) and 4,[5],12,i:- (n = 5; 9.3%) (Table 2). In the animals/meat sources, the most common ESBL-producing serotypes were Albany (n = 15; 36.6%), Heidelberg (n = 6; 14.6%), and Agona (n = 4; 9.8%) (Table 3). Overall, Salmonella Heidelberg was the most commonly detected ESBL-producing serotype (n = 22; 23.2%) in the study.
A wider diversity of ESBL enzymes were found in human sources (9 alleles) than in animals/meat sources (4 alleles). In human-source Salmonella, the most common ESBLs were blaCTX-M-65 (n = 18, 33.3%), blaSHV-2 (n = 12, 22.2%), and blaCTX-M-55 (n = 6, 11.1%) (Table 2). Human-source Salmonella also carried blaCTX-M-1, blaCTX-M-3, blaCTX-M-9, blaCTX-M-14, and blaCTX-M-15 and blaSHV-5 at lower frequencies. Most animals/meat-source isolates contained either blaCTX-M-1 (n = 19, 46.3%) or blaSHV-2 (n = 15, 36.6%); the remaining isolates contained either blaSHV-12 (n = 6) or blaCTX-M-55 (n = 1) (Table 3). Thus, the blaSHV-2 gene was detected in 20% of ESBL Salmonella from human sources and in 33.3% of ESBL Salmonella from animals/meat sources. Except for isolate 12-0820, all blaSHV-2 isolates carried a known L31Q substitution, which is sometimes referred to as blaSHV-2a.
Drug Resistance Profiles
Resistance to ampicillin, streptomycin, sulfisoxazole, and tetracycline (ASSuT) was commonly observed in ESBL-producing Salmonella isolates from both sources (Table 4). For human source isolates, 25 (46.2%) demonstrated the ASSuT and chloramphenicol resistance pattern. For animals/meat sources, 12 (29.3%) isolates displayed the ASSuT and gentamicin resistance pattern. Although blaSHV and blaCTX-M alleles conferred ceftriaxone resistance (MIC resistance breakpoint >4 mg/L), isolates that had blaSHV showed MICs of 4–8 mg/L, and isolates that had blaCTX-M showed 8-fold higher MICs of 32–64 mg/L. Intermediate or outright resistance to ciprofloxacin was frequently observed in human-source ESBL-producing Salmonella (n = 31, 57.4%) but not in animals/meat sources.
There was general agreement between resistance phenotypes and genotypes (Table 4). CMY-2, but not ESBLs, confer resistance to clavulanic acid; 6 animals/meat isolates had resistance to amoxicillin/clavulanic acid and all contained blaCMY-2. One human-derived isolate of Salmonella 4,[5],12:i:- had the mobile colistin resistance gene mcr-3.2, along with blaCTX-M-55 and other resistance genes conferring resistance to 8 classes of antimicrobial drugs; the isolate had been described (25). Agreement between phenotype and genotype for gentamicin was lower; 12/25 isolates that had predicted gentamicin resistance contained the aac (3)-IVa gene conferring MICs of 4–8 mg/L, which is 1 or 2 dilutions below the resistance breakpoint of MIC >16 mg/L and accounted for most of the discrepancy. In general, disagreements between susceptibility phenotype and genotype might be caused by resistance genes or mutations that are currently unknown.
Phylogenomic Relatedness of Strains
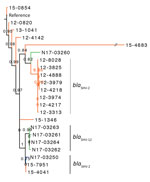
We created maximum-likelihood phylogenetic trees based on SNVs in the core genome for each serotype. The Salmonella Heidelberg phylogenetic tree showed that ESBL-producing isolates from human and animals/meat sources were genetically distinct, with some exceptions (Figure 1). Closely related Salmonella Heidelberg (defined as <20 SNVs) carrying blaSHV-2 were identified from chicken thighs (N17-03250 isolated in western Canada during 2013) and humans (15-7951 and 15-4041 isolated in western Canada during 2015); these isolates differed by only 1–7 SNVs. The branch support for these 3 isolates was not strong (SH-like value 0.76). However, the isolates carried similar type A IncI1-blaSHV-2 plasmids (described in the ESBL Plasmids Section). The 3 isolates carrying blaSHV-2 were also genetically similar to 4 isolates carrying blaSHV-12, differing by only 9–14 SNVs.
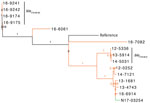
The phylogenetic tree for Salmonella Typhimurium and closely related serovars Salmonella 4,5,12,i:- and Salmonella Typhimurium var. O:5 showed that isolates from human and animals/meat sources were genetically distinct with 1 potential exception (Figure 2). Among Salmonella 4,[5],12,i:- carrying blaCTX-M-55, 1 clinical isolate from a sick pig (N17-03254 isolated in central Canada during 2015) differed by 55–80 SNVs from 3 human isolates (13-1681, 13-4743, and 16-6914, isolated in central and western Canada during 2013 and 2016) (Figure 2). More epidemiologic studies are needed to interpret whether 55–80 SNVs indicate genetic relatedness. However, the clustering of isolates from human and pig was strongly supported (SH-like value 0.99).
Salmonella Agona and Salmonella Infantis were the only other serotypes in which ESBL-producing isolates were detected in both sources. Phylogenetic trees of Salmonella Agona did not show evidence of transmission between animals/meat and humans because isolates from the 2 sources differed by >77 SNVs. The United States and other countries have noted the emergence of Salmonella Infantis carrying CTX-M-65 on the plasmid of emerging Salmonella Infantis in humans and food animals, especially in poultry (26). For comparison, we included genome sequences of Salmonella Infantis from human, food animals, and retail chicken from the study by Tate et al. in our phylogenetic tree (26). The major clade of the tree comprised CTX-M-65‒containing isolates from both studies whereby isolates differed by 1‒53 SNVs. Three isolates from human sources in Canada collected during 2016 were closely related to isolates from retail chicken, chicken at slaughter, and dairy cow at slaughter collected in the United States during 2014–2015, differing by only 4–13 SNVs and clustering on a strongly supported branch (SH-like value 1.0) (Figure 3). One isolate each from Canada from a cat (N17-03255) carrying SHV-2 and a human (15-8465) carrying CTX-M-3 did not cluster with the CTX-M-65-containing isolates.
ESBL Plasmids
Although the ESBL serotypes were mostly different between humans and animals/meat isolates, it is possible that ESBL plasmids were similar because plasmids can be transmissible between serotypes. We produced complete plasmid sequences for a subset of isolates.
The blaSHV-2 genes from human sources were all from Salmonella Heidelberg, and the animals/meat isolates were from a variety of serotypes, including Agona, Anatum, Brandenburg, California, Derby, Heidelberg, Infantis, and Ohio (Table 2). All blaSHV-2 genes were carried on IncI plasmids, which we categorized into 3 types (types A, B, and C) on the basis of their resistance gene profiles and overall genetic content (Figure 4). Similar blaSHV-2 plasmids were found in isolates from humans and animals/meat (>95% nucleotide identity over >90% plasmid length).
The type A plasmids carried blaSHV-2 and tetA resistance genes and were found in humans (n = 10), chicken(s) (n = 2, one each from meat and animal), and pig (n = 1 from animal) (Figure 4). Three of the type A plasmids were from Salmonella Heidelberg isolates that differed by only 1–7 SNVs (N17-03250 isolated from chicken thighs during 2013; and 15-7951 and 15-4041 isolated from humans during 2015). Thus, these 3 isolates were genetically closely related and contained similar plasmids (99.9% nucleotide identity over 91% of the length of the plasmid). The type B plasmids carried aac (3)-VIa, ant(3′′)-Ia, blaSHV-2, and sul1 and were found in a human (n = 1), a domestic cat (n = 1), and turkey/turkeys (n = 5). The type B plasmid from a human (12-0820 in Salmonella Heidelberg) was most similar to the plasmid from the domestic cat (N17-03255 in Salmonella Infantis) with 99.9% nucleotide identity over 93% of the length of the plasmid. Finally, the type C plasmids carried aadA1, blaSHV-2, dfrA1, and sul1. The type C plasmids were isolated from pigs on farms and from sick pigs.
The blaCTX-M-1‒containing plasmid was the most common ESBL in animals/meat isolates and was occasionally observed in human isolates. One blaCTX-M-1 plasmid from serotype Salmonella Worthington identified from a pig (N16-01063 isolated in western Canada during 2013) and all blaCTX-M-1 plasmids from Salmonella Heidelberg (n = 3) and Salmonella Typhimurium (n = 1) from human sources in various years were carried on similar IncI plasmids (Figure 5, panel A). Of 119 coding sequences on the blaCTX-M-1 IncI plasmid from pig (N16-01063), all but 1 coding DNA sequence was present on the human plasmids. The plasmid had only 75% nucleotide identity to the previously reported R64 reference plasmid (27). However, in the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov), there were matches to E. coli plasmids (e.g., accession no. CP007651.1).
In the remainder of animals/meat isolates harboring blaCTX-M-1, the gene was carried on an IncN plasmid (Figure 5, panel B). IncN blaCTX-M-1 plasmids were similar between isolates except for N16-01061, which was missing ≈20 kb. A representative blaCTX-M-1 IncN plasmid from isolate N17-03257 had >99.5% nucleotide identity to plasmids from E. coli O16:H48 (accession no. CPO34186.1) and Salmonella Bredeney (accession no. CPO43184.1). The blaCTX-M-55 was found on IncN plasmids in Salmonella 4,[5],12:i:- in 1 isolate each from human and animals/meat source. However, of 291 coding DNA sequences on the plasmid from a pig isolate (N17-03254), only 242 (83%) were present on the plasmid from the human isolate (13-1681) (Figure 5, panel C).
The most common combination of ESBL Salmonella serotype and allele in humans in this study was Salmonella Infantis carrying blaCTX-M-65 (n = 14). The blaCTX-M-65 IncFIB plasmid was almost identical to a plasmid that the National Antimicrobial Resistance Monitoring System (NARMS) has reported to be emerging in the United States in humans and chickens (26,28). A representative closed plasmid from isolate 15-4113 in this study had 99.1% nucleotide identity with the reported NARMS plasmid (accession no. NZ_CP016407). Of 14 isolates of Salmonella Infantis carrying CTX-M-65 detected in Canada, 9 isolates contained 11 antimicrobial resistance determinants: aac (3)-IVa, ant(3′′)-Ia, aph(3′′)-Ia, aph (4)-Ia, blaCTX-M-65, dfrA14, floR, fosA3, gyrA (D87Y), sul1, and tet(A) conferring reduced susceptibility or resistance to gentamicin, streptomycin, ampicillin, ceftriaxone, trimethoprim, chloramphenicol, ciprofloxacin, nalidixic acid, sulfisoxazole, and tetracycline. Gene fosA3 probably confers reduced susceptibility to fosfomycin, but this antimicrobial drug was not tested. All resistance determinants except for gyrA (D87Y) were carried on the representative closed plasmid. Two isolates lacked fosA3, 2 lacked fosA3 and aph (4)-Ia, and 1 lacked fosA3, ant(3′′)-Ia and aph(3′)-Ia.
Health Canada has classified third-generation and fourth-generation cephalosporins as Category I (high importance) antimicrobial drugs based on their role in human medicine. However, Category I antimicrobial drugs are still used in food animals with a veterinary prescription with some restrictions (8,29). The frequency of ESBL-producing Enterobacterales continues to increase in humans, especially in E. coli and K. pneumoniae (30). In this study, the frequency of recovery of ESBL-producing Salmonella (i.e., no. ESBL-producing isolates/total no. isolates) during 2012‒2016 was low (0.35% from humans and 0.31% from animals/meat). Recent studies in China have reported a much higher frequency of recovery of ESBL-producing Salmonella from food (9.7%) and food animals (17.7%) (31,32).
In our study, 76% of ESBL-producing Salmonella causing human infections and 49% from animals/meat isolates harbored CTX-M, and the remainder harbored SHV. During the 1990s, global outbreaks of ESBL-producing Enterobacterales were mainly caused by K. pneumoniae carrying SHV and TEM enzymes; since then, CTX-M enzymes have increased rapidly and are now the most common ESBL enzymes (33).
For ESBL-related infections in humans, CTX-M-14 and CTX-M-15 are the most common ESBLs in E. coli (10,33). However, in our study, these 2 alleles were infrequently observed in Salmonella. In our study, Salmonella Infantis carrying blaCTX-M-65 was the most common ESBL-producer detected from human infections in Canada. In the United States, although ESBL-producing Salmonella are rare, Salmonella Infantis carrying blaCTX-M-65 is emerging in human infections and in poultry (26,28). Salmonella Infantis containing blaCTX-M-65 is also emerging in humans and poultry in other countries, including Italy, England and Wales, Israel, Peru, and Ecuador (34–38).
The blaCTX-M-65 is carried on a large IncFIB plasmid termed plasmid of emerging Salmonella Infantis along with other resistance determinants. The blaCTX-M-65 plasmid detected in Canada was almost identical to the IncFIB blaCTX-M-65 plasmid that was reported in the United States (25,27). This plasmid is especially concerning because it is transferrable and it carries <10 genes encoding resistance to ampicillin, ceftriaxone, chloramphenicol, gentamicin, nalidixic acid, streptomycin, sulfisoxazole, tetracycline, and trimethoprim. Salmonella Infantis containing blaCTX-M-65 was found exclusively from human infections in our study, but human isolates from Canada collected during 2016 were closely related to isolates from humans, chicken, and dairy cow from the United States that were collected during 2014. The human cases in Canada might have been imported through retail sources, or travel, or might have been acquired from domestic food commodities that were not sampled by CIPARS during the study. Continued surveillance is needed to detect potential emergence of ESBLs in food animals, meat, and other food commodities in Canada.
In animals/meat isolates, blaCTX-M-1 was the most common allele detected and is the most common allele in animals/meat sources from western Europe (33,39). The allele blaCTX-M-27 is emerging in China and Vietnam and was detected in the United States, but this allele was not observed in Canada during this study (40,41).
The Salmonella ESBL alleles and serotypes were mostly different between humans and domestic animals/meat sources during the study period. A meta-analysis of risk factors for fecal ESBL colonization identified recent antimicrobial drug use and international travel as the 2 major risk factors (42). CIPARS does not collect information on human travel or imported foods, but these factors might contribute to ESBLs in humans in Canada. Although ESBLs were not detected in typhoidal Salmonella during the study period, several cases of extensively drug resistant Salmnella Typhi containing blaCTX-M-15 were imported into Canada during 2018 and 2019 by patients who had traveled to Pakistan, where a large outbreak is ongoing (43). Pets might be another reservoir of ESBL-producing bacteria. Although CIPARS examined only 22 pet samples in this study, we detected a blaSHV-2 plasmid in a cat that was almost identical to a plasmid from a human isolate.
In summary, ESBL-producing E. coli and K. pneumoniae are a healthcare challenge because treatment options are limited (30). Although the frequency of recovery of ESBL-producing Salmonella was low in this study, it is essential to continue surveillance because extended-spectrum cephalosporins are a major treatment option for serious or invasive Salmonella infections.
Dr. Bharat is the Head of Mycology and Molecular Enteric Antimicrobial Resistance at the National Microbiology Laboratory within the Public Health Agency of Canada, Winnipeg, Manitoba, Canada. Her primary research interests are antimicrobial drug resistance in Salmonella sp. and E. coli, and antifungal drug resistance in Candida spp.
Acknowledgments
We thank the Genomics Core Facility and the Bioinformatics Core Facility at the National Microbiology Laboratory for providing support with WGS and bioinformatics.
This study was supported by internal funds from the Public Health Agency of Canada.
References
- GBD 2017 Non-typhoidal Salmonella invasive disease collaborators. The global burden of non-typhoidal Salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:1312–24.
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al.; GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. DOIPubMedGoogle Scholar
- Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65:1963–73. DOIPubMedGoogle Scholar
- Bush K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2018;62:e01076–18. DOIPubMedGoogle Scholar
- Karaiskos I, Giamarellou H. Carbapenem-sparing strategies for ESBL producers: when and how. Antibiotics (Basel). 2020;9:61. DOIPubMedGoogle Scholar
- Hernando-Amado S, Coque TM, Baquero F, Martínez JL. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat Microbiol. 2019;4:1432–42. DOIPubMedGoogle Scholar
- Chicken Farms of Canada. Preventative use of category I antibiotics in the poultry and egg sectors, 2013 [cited 2020 Dec 19]. https://www.chickenfarmers.ca/antibiotics
- Health Canada. Responsible use of medically important antimicrobials in animals. 2020. p. 1‒15 [cited 2020 Dec 18]. https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/actions/responsible-use-antimicrobials.html
- Denisuik AJ, Karlowsky JA, Adam HJ, Baxter MR, Lagacé-Wiens PRS, Mulvey MR, et al.; Canadian Antimicrobial Resistance Alliance (CARA) and CANWARD. Dramatic rise in the proportion of ESBL-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates identified in Canadian hospital laboratories from 2007 to 2016. J Antimicrob Chemother. 2019;74(Suppl 4):iv64–71. DOIPubMedGoogle Scholar
- Kazmierczak KM, de Jonge BLM, Stone GG, Sahm DF. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013-17. J Antimicrob Chemother. 2020;75:1165–73. DOIPubMedGoogle Scholar
- Toy T, Pak GD, Duc TP, Campbell JI, El Tayeb MA, Von Kalckreuth V, et al. Multicountry distribution and characterization of extended-spectrum β-lactamase-associated Gram-negative bacteria from bloodstream infections in Sub-Saharan Africa. Clin Infect Dis. 2019;69(Suppl 6):S449–58. DOIPubMedGoogle Scholar
- Sjölund-Karlsson M, Howie RL, Blickenstaff K, Boerlin P, Ball T, Chalmers G, et al. Occurrence of β-lactamase genes among non-Typhi Salmonella enterica isolated from humans, food animals, and retail meats in the United States and Canada. Microb Drug Resist. 2013;19:191–7. DOIPubMedGoogle Scholar
- Sjölund-Karlsson M, Rickert R, Matar C, Pecic G, Howie RL, Joyce K, et al. Salmonella isolates with decreased susceptibility to extended-spectrum cephalosporins in the United States. Foodborne Pathog Dis. 2010;7:1503–9. DOIPubMedGoogle Scholar
- Sjölund-Karlsson M, Howie R, Krueger A, Rickert R, Pecic G, Lupoli K, et al. CTX-M-producing non-Typhi Salmonella spp. isolated from humans, United States. Emerg Infect Dis. 2011;17:97–9. DOIPubMedGoogle Scholar
- CIPARS. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), 2018: design and methods. 2020. p. 1–57 [cited 2022 May 3]. https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars/cipars-reports/2018-annual-report-design-methods.html
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing (M100–ED29). Wayne (PA); The Institute; 2019. p. 1–321 [cited 2022 May 3]. https://clsi.org/media/2663/m100ed29_sample.pdf
- Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg Infect Dis. 2011;17:103–6. DOIPubMedGoogle Scholar
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–77. DOIPubMedGoogle Scholar
- Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLOS Comput Biol. 2017;13:
e1005595 . DOIPubMedGoogle Scholar - Bharat A, Petkau A, Avery BP, Chen JC, Folster JP, Carson CA, et al. Correlation between phenotypic and in silico detection of antimicrobial resistance in Salmonella enterica in Canada using Staramr. Microorganisms. 2022;10:292. DOIPubMedGoogle Scholar
- Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–4. DOIPubMedGoogle Scholar
- Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72:2764–8. DOIPubMedGoogle Scholar
- Petkau A, Mabon P, Sieffert C, Knox NC, Cabral J, Iskander M, et al. SNVPhyl: a single nucleotide variant phylogenomics pipeline for microbial genomic epidemiology. Microb Genom. 2017;3:
e000116 . DOIPubMedGoogle Scholar - Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–21. DOIPubMedGoogle Scholar
- Mulvey MR, Bharat A, Boyd DA, Irwin RJ, Wylie J. Characterization of a colistin-resistant Salmonella enterica 4,[5],12:i:- harbouring mcr-3.2 on a variant IncHI-2 plasmid identified in Canada. J Med Microbiol. 2018;67:1673–5. DOIPubMedGoogle Scholar
- Tate H, Folster JP, Hsu C-H, Chen J, Hoffmann M, Li C, et al. Comparative analysis of extended-spectrum-β-lactamase CTX-M-65‒producing Salmonella enterica serovar Infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrob Agents Chemother. 2017;61:e00488–17. DOIPubMedGoogle Scholar
- Sampei G, Furuya N, Tachibana K, Saitou Y, Suzuki T, Mizobuchi K, et al. Complete genome sequence of the incompatibility group I1 plasmid R64. Plasmid. 2010;64:92–103. DOIPubMedGoogle Scholar
- Brown AC, Chen JC, Watkins LKF, Campbell D, Folster JP, Tate H, et al. CTX-M-65 Extended-spectrum β-lactamase‒producing Salmonella enterica serotype Infantis, United States. Emerg Infect Dis. 2018;24:2284–91. DOIPubMedGoogle Scholar
- Health Canada. Categorization of antimicrobial drugs based on importance in human medicine. 2020. p. 1‒9 [cited 2020 Dec 22]. https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html
- Peirano G, Pitout JDD. Extended-spectrum β-lactamase‒producing Enterobacteriaceae: update on molecular epidemiology and treatment options. Drugs. 2019;79:1529–41. DOIPubMedGoogle Scholar
- Zhang C-Z, Ding X-M, Lin X-L, Sun R-Y, Lu Y-W, Cai R-M, et al. The emergence of chromosomally located blaCTX-M-55 in Salmonella from foodborne animals in China. Front Microbiol. 2019;10:1268. DOIPubMedGoogle Scholar
- Wang W, Zhao L, Hu Y, Dottorini T, Fanning S, Xu J, et al. Epidemiological study on prevalence, serovar diversity, multidrug resistance, and CTX-M-type extended-spectrum β-lactamases of Salmonella spp. from patients with diarrhea, food of animal origin, and pets in several provinces of China. Antimicrob Agents Chemother. 2020;64:10. DOIPubMedGoogle Scholar
- Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72:2145–55. DOIPubMedGoogle Scholar
- Franco A, Leekitcharoenphon P, Feltrin F, Alba P, Cordaro G, Iurescia M, et al. Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS One. 2015;10:
e0144802 . DOIPubMedGoogle Scholar - Alba P, Leekitcharoenphon P, Carfora V, Amoruso R, Cordaro G, Di Matteo P, et al. Molecular epidemiology of Salmonella Infantis in Europe: insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microb Genom. 2020;6:
e000365 . DOIPubMedGoogle Scholar - Lee WWY, Mattock J, Greig DR, Langridge GC, Baker D, Bloomfield S, et al. Characterization of a pESI-like plasmid and analysis of multidrug-resistant Salmonella enterica Infantis isolates in England and Wales. Microb Genom. 2021;7:
000658 . DOIPubMedGoogle Scholar - Cartelle Gestal M, Zurita J, Paz Y Mino A, Ortega-Paredes D, Alcocer I, Alcocer I, et al. Characterization of a small outbreak of Salmonella enterica serovar Infantis that harbour CTX-M-65 in Ecuador. Braz J Infect Dis. 2016;20:406–7. DOIPubMedGoogle Scholar
- Martínez-Puchol S, Riveros M, Ruidias K, Granda A, Ruiz-Roldán L, Zapata-Cachay C, et al. Dissemination of a multidrug resistant CTX-M-65 producer Salmonella enterica serovar Infantis clone between marketed chicken meat and children. Int J Food Microbiol. 2021;344:
109109 . DOIPubMedGoogle Scholar - de Been M, Lanza VF, de Toro M, Scharringa J, Dohmen W, Du Y, et al. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet. 2014;10:
e1004776 . DOIPubMedGoogle Scholar - Zhang W-H, Lin X-Y, Xu L, Gu X-X, Yang L, Li W, et al. CTX-M-27 producing Salmonella enterica serotypes Typhimurium and Indiana are prevalent among food-producing animals in China. Front Microbiol. 2016;7:436. DOIPubMedGoogle Scholar
- Elnekave E, Hong SL, Lim S, Hayer SS, Boxrud D, Taylor AJ, et al. Circulation of plasmids harboring resistance genes to quinolones and/or extended-spectrum cephalosporins in multiple Salmonella enterica serotypes from swine in the United States. Antimicrob Agents Chemother. 2019;63:901. DOIPubMedGoogle Scholar
- Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis. 2016;63:310–8. DOIPubMedGoogle Scholar
- Eshaghi A, Zittermann S, Bharat A, Mulvey MR, Allen VG, Patel SN. Importation of extensively drug-resistant Salmonella enterica serovar Typhi cases in Ontario, Canada. Antimicrob Agents Chemother. 2020;64:
e570 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 15, 2022
Table of Contents – Volume 28, Number 7—July 2022
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Amrita Bharat, Public Health Agency of Canada, National Microbiology Laboratory, H5050, 1015 Arlington St, Winnipeg, MB R3E 3R2, Canada
Top