Volume 29, Number 11—November 2023
Research
Neurologic Effects of SARS-CoV-2 Transmitted among Dogs
Abstract
SARS-CoV-2 induces illness and death in humans by causing systemic infections. Evidence suggests that SARS-CoV-2 can induce brain pathology in humans and other hosts. In this study, we used a canine transmission model to examine histopathologic changes in the brains of dogs infected with SARS-CoV-2. We observed substantial brain pathology in SARS-CoV-2–infected dogs, particularly involving blood–brain barrier damage resembling small vessel disease, including changes in tight junction proteins, reduced laminin levels, and decreased pericyte coverage. Furthermore, we detected phosphorylated tau, a marker of neurodegenerative disease, indicating a potential link between SARS-CoV-2–associated small vessel disease and neurodegeneration. Our findings of degenerative changes in the dog brain during SARS-CoV-2 infection emphasize the potential for transmission to other hosts and induction of similar signs and symptoms. The dynamic brain changes in dogs highlight that even asymptomatic individuals infected with SARS-CoV-2 may develop neuropathologic changes in the brain.
Since SARS-CoV-2 was first reported in late 2019, infection has been observed primarily in humans; however, animals of various species have also been infected, partially because their angiotensin-converting enzyme 2 (ACE2) receptor is very similar to that of humans. Infected animals show clinical signs similar to those of humans, raising concerns about potential transmission of the virus between humans and animals (1,2). SARS-CoV-2 infection in dogs and cats affects the lungs and leads to pathologic changes (Figure 1). However, whether similar pathologic manifestations occur in the brain, as observed in humans, remains unclear.
Close cohabitation of dogs and humans, and their high genetic similarity, has prompted investigations into dogs’ susceptibility to SARS-CoV-2 infection (3,4). Wild-type SARS-CoV-2 infection in dogs can induce formation of neutralizing antibodies, and low viral titers in dogs demonstrate seroconversion (5,6). Mutant strains of SARS-CoV-2 in dogs cause histopathologic changes in lung tissues and increased expression of muscle damage markers in the blood (7). ACE2 in dogs can bind to the receptor-binding domain of SARS-CoV-2, implying the possibility of cross-species transmission between humans and dogs (8). Genetic and epidemiologic studies have reported animal-to-human transmission of SARS-CoV-2 (9).
Reportedly, SARS-CoV-2 can cause neurologic signs and symptoms (e.g., headache, fatigue, and cognitive dysfunction) in human patients. Several cohort studies report strong correlations between SARS-CoV-2 and neurologic signs/symptoms (10–13). Furthermore, cortical thickness is reduced in SARS-CoV-2–infected patients, suggesting that SARS-CoV-2 can induce pathologic changes in the brain, which may be linked to the functional deficits noted in those patients. Considering the number of patients infected with SARS-CoV-2, the neurologic signs can lead to a potential wave of neurodegenerative diseases, which could pose an immense burden on society.
The etiology of SARS-CoV-2–induced neuropathologic changes is still elusive. However, clinical and experimental reports suggest that vascular damage and the resultant immune responses in the brain may be a major factor (13–16). Magnetic resonance imaging has detected white matter hyperintensities in SARS-CoV-2–infected patients, indicating damage to the blood–brain barrier (BBB) in this region and that potentially demyelinating pathologic changes can be induced (13). Other studies have revealed signs of neuroinflammatory responses, including activation of microglial cells and astrocytes (14,15). Moreover, damage to the brain vasculature and defects in the coagulation system have been demonstrated (16). The characteristic pathologies observed in human patients (e.g., vascular damage, demyelination, and neuroinflammatory responses) have also been observed in humanized mouse models.
We used a canine transmission model to investigate the susceptibility of dogs to SARS-CoV-2, specifically the Delta variant. The dogs were housed in a Biosafety Level 3 animal facility at Konkuk University Laboratory, Seoul, South Korea, where temperature, humidity, and light were carefully controlled. The study was approved by the Animal Research Center under the supervision of the Institutional Animal Care and Use Committee (accreditation no. KU22065) and the Institutional Biosafety Committee (accreditation no. KUIBC-2022-06) at Konkuk University. The absence of SARS-CoV-2 RNA and SARS-CoV-2 antibodies in dog serum was confirmed.
Considering that SARS-CoV-2 infections cause neurologic effects in human and human ACE2 transgenic mice, and typically follow respiratory system infection, we used models mimicking the natural infection route. We intranasally infected dogs with the Delta variant, and virus subsequently was transmitted to contact dogs. We assessed detection of viruses in the brain and damage to the integrity of the BBB as well as activation of neuroimmune responses in the brain. To test whether SARS-CoV-2 can indeed induce neuropathologic changes in the brain, we also assessed further patterns of demyelination and axonal damage. We describe our methods here in brief; details are provided in the Appendix,
We purchased fifteen 6-month-old female conventional beagles from Orient Bio, South Korea (http://www.orient.co.kr) and classified them into 3 groups: control (n = 3), infection (n = 6), and contact (n = 6). The dogs in the infection and contact groups were housed in 6 cages, each measuring 800 mm wide × 900 mm deep × 800 mm high.
To mimic natural infection, we implemented 2 infection models: intranasally inoculated dogs and dogs infected via horizontal transmission. We anesthetized 6 dogs in the infection group with 0.3 mg/kg of alfaxalone and then intranasally inoculated each dog with 105 PFU of SARS-CoV-2 Delta variant. After the dogs regained consciousness and acclimated to the environment, each infected dog was placed in a cage with a dog from the contact group. To control for any potential effects of the inoculation procedure or medium, we intranasally inoculated dogs in the control group with 500 µL of Dulbecco Modified Eagle Medium. Veterinarians visually examined the dogs for clinical signs, including neurologic signs.
With the infection model established, we next investigated the neuropathologic changes in the brain. First, we confirmed the existence of viral particles in the brain because it is logical to consider that viral particles can migrate to and replicate in the brain, which would directly damage the brain. To confirm the presence of viral nucleic acid we used quantitative reverse-transcription PCR, and to confirm the presence of viral particles we used immunofluorescence assays.
For use in additional experiments, at 4, 7, 11, 14, 18, 21, 25, 28, 32, and 35 days postinfection (dpi), we collected nasopharyngeal, oropharyngeal, and fecal swab and blood samples from all dogs while they were under sedation. At each timepoint in the early (10, 12, and 14 dpi) and late (38, 40, and 42 dpi) periods of infection, dogs were sedated and euthanized by intravenous injection of supersaturated KCl and performed necropsies (only 1 infected and 1 contact dog could be necropsied at each timepoint because of logistical constraints).
Samples underwent quantitative reverse transcription PCR, immunohistochemistry, immunofluorescence staining, ELISA, and plaque reduction neutralization test, as indicated (Appendix). We conducted all experiments in triplicate and express results as mean ±SD. We plotted dose-response curves, and we performed Student t-tests by using Prism 8.0.1 (Graphpad software, https://www.graphpad.com). We set statistical significance at p<0.05.
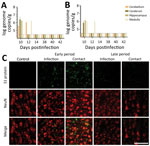
We detected no significant changes in objective measurements of the dogs (body weight and temperature). No dogs exhibited apparent neurologic signs or respiratory signs resembling COVID-19 (Appendix).
Pathologic Changes in the Integrity of the BBB
In this study, we detected viral RNA in the brain during the early infection period only, not during the late infection period (Figure 2, panel A). We confirmed colocalization of the viral particles with neuronal cells by using an immunofluorescence assay with an antibody specific to the spike protein of the virus. As for viral RNA, we also detected viral particles only during the early infection period (Figure 2, panel B). Our observations indicate that SARS-CoV-2 may infect the brain during the early infection period and may be cleared by the later infection period.
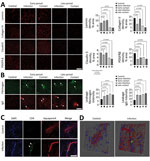
Various reports suggest damaged brain vasculature in SARS-CoV-2–infected human patients, which is reported to be associated with the influx of peripheral molecules and activation of immune responses in the brain (13–16). In our study, we tested the pathologic changes in the canine brain vasculature by using an immunofluorescence assay with antibodies specific to the BBB compartments. For dogs infected with the virus, pathologic alterations in the BBB structure were noted, showing decreased signals of matrix proteins (laminin and collagen IV) and tight junction protein (claudin 5) (Figure 3, panel A). In dogs of both groups, those phenomena were prominently observed during late rather than early infection. In addition, PDGFR-β densities, which are markers for pericytes, were decreased in dogs of both groups during the early and late periods, indicating that the cellular components of the BBB were damaged by viral infection. Moreover, the infiltrations of fibrinogen and IgG were found in the parenchyma of the brain, indicating that viral infections breached the functional integrity of the BBB (Figure 3, panel B). Last, infiltration of CD4+ T cells was found in the brain by trespassing into the BBB matrix protein layer, suggesting severe damage to the BBB integrity and subsequent recruitment of these cells into the brain (Figure 3, panels C, D). Those observations indicate that SARS-CoV-2 infection could induce pathologic changes in the structural and functional integrity of the BBB. Such changes may allow entry of peripheral molecules and immune cells into the brain parenchyma during the early infection period. Collectively, the pathologic changes concur with the typical signs of small vessel disease (SVD).
Neuroinflammatory Responses in the Brain
When we tested whether SARS-CoV-2–induced damage of the BBB can induce neuroinflammatory responses, we stained brain sections with markers for glial activation, including glial fibrillary acidic protein and Iba-1, which are markers for activated astrocytes and microglial cells. Glial fibrillary acidic protein showed a statistically significant increase in the brain white matter of dogs in the infection and contact groups at the early and late periods, suggesting potential proinflammatory conditions in the brain (Figure 4, panel A). When we tested activation of microglial cells (another major component of innate immune responses in the brain) by staining brain sections with an antibody specific to Iba-1 (a marker of activated microglial cells), we observed a significant increase in Iba-1–positive signals in the brain white matter of dogs from both groups during the early and late periods (Figure 4, panel B). However, we did not observe such increases in the gray matter, suggesting that the microglial cell activations were specific for white matter (Figure 4, panel C). Of note, we observed activated microglial cell clustering in several spots in the white matter from dogs in both groups. Overall, those observations suggest that BBB disruption mediated by infection with SARS-CoV-2 could elicit neuroinflammatory responses and further contribute to the progression of neurodegenerative pathology in canine brains.
Typical Signs of SVD-mediated Axonopathy
The early signs of SVD-mediated brain neurodegeneration are pathologic changes in axons and demyelination. To verify whether SARS-CoV-2 can induce these pathologic changes, we stained brain sections with antibodies against the neurofilament light chain (NFL). The intensities of NFL staining were significantly lower in the brain white matter of dogs in both groups at the early and late periods than in the uninfected control dogs (Figure 5, panels A, B). In addition, the pathologic changes in the structure and integrity of the NFL were evidenced by swelling and irregularity. Decreased NFL intensities were more severe in perivascular regions, as typical signs of SVD-mediated axonopathy.
Because demyelination is another hallmark of SVD, we assessed demyelination by using fluomyelin, a fluorescent marker for myelin. We observed a significantly lower intensity of fluomyelin in the brain white matter of dogs in the infection and contact groups during the early and late periods; NFL patterns were similar in both groups (Figure 5, panels C, D). Those changes were more evident in the perivascular area, identical to the NFL lesions. The axonopathy-like changes observed at the perivascular area of the white matter could be a consequence of SVD induction.
Pathologic Signs of Neurodegenerative Diseases, Represented by Tauopathy
To assess production of Aβ aggregation and test whether SVD-induced neuropathologic changes can further cause neurodegenerative signs, we stained brains with an amyloid β (6E10) antibody. However, we did not observe formation of Aβ aggregates in the brains of any dogs during the early or late periods (Appendix Figure 10, panel A). Next, to assess tauopathy in the virus-infected brains, we stained brain sections with different types of phosphorylated tau. Using a p-Tau 181 antibody, we did not observe positive signals for phosphorylated tau in any dogs (Appendix Figure 10, panel B). However, we detected phosphorylation of tau at Ser202/Thr205 by using an AT8 antibody in the brains of dogs in the infection group during the early period and dogs in the infection and contact groups during the late period. Those results suggest that SARS-CoV-2 infection could induce accumulation of the pathologic form of tau in a site-specific manner (Figure 5, panel E). Last, we used the number of neuronal cells to determine whether those pathologic neurodegenerative signatures are associated with loss of neuronal cells. We did not observe statistically significant changes in the number of cortical neurons in the brains of dogs from the infection and contact groups during the early period (Figure 5, panels F–H). However, we observed decreased numbers of neuronal cells in the infection and contact groups during the late period. Therefore, degenerative changes such as tauopathy and decreased numbers of neuronal cells in the virus-infected brains seemed to be induced after elicitation of pathologic drivers, including BBB damage, glial activation, and axonopathy, as consequences of SVD.
Overall, our study demonstrates solid experimental evidence that SARS-CoV-2 can infect dogs and be transmitted to others by direct contact, producing pathologic brain changes even without prominent signs. Pathologic changes in the lung and brain were observed in dogs of both groups, providing additional evidence of virus transmission. Of note, SARS-CoV-2 infection has been reported to cause long-term pathologic effects even after the virus is cleared from the main organs of the body (17). Our study provides evidence that SARS-CoV-2 infection can damage the brain as well as the lungs in dogs at early and later stages of infection, suggesting a high potential for a long-lasting COVID-19–like syndrome to develop in affected dogs.
We detected SARS-CoV-2 in secretions from the nasopharynx and oropharynx of dogs in both the infection and contact groups, albeit at a low percentage. Remarkably, we found that the viral titers were higher in the nasal and oral mucosa of dogs in the contact group than in those in the infection group. That finding could be attributed to the role of the nasal and oral cavities as routes of virus entry for the contact group, resulting in higher replication of the virus at these entry points (18). We observed that during the early stages of infection, dogs in the contact group exhibited more severe inflammatory responses in the trachea and bronchioles than did those in the infection group. Those findings are consistent with results of previous studies that have shown that contact transmission can result in higher levels of virus titers and lead to more rapid onset of pathologic changes in the upper respiratory tract (19,20).
Seroconversion in dogs after SARS-CoV-2 infection was observed as early as 4 dpi; the rapid seroconversion may be associated with the absence of clinical signs (21). Antibody levels peaked a few days later in dogs in the contact group than in dogs in the infection group, suggesting later virus transmission from the virus-infected dogs. Neutralizing antibody titers against SARS-CoV-2 strongly correlate with antibody titers of the spike protein, highlighting the spike protein as a crucial target for the humoral immune response (R value >0.7; p< 0.001).
The lung alveolar septum of infected dogs was thickened overall, caused by infiltration of immune cells that indicate interstitial pneumonia (e.g., mononuclear cells, neutrophils, and macrophages) (22,23), associated with the presence of neutrophil elastase–positive cells and Iba-1–positive cells. Neutrophil elastase and Iba-1 levels increase in response to SARS-CoV-2 infection (22,24). Neutrophil elastase–positive and Iba-1–positive cells were found infiltrated around the blood vessels, indicating perivasculitis in SARS-CoV-2 infected dogs as in other hosts (22,25).
Brain damage induced by viral infection has been reported from various nonneurotrophic viruses, including HIV (17,26). Contrary to previous beliefs, accumulating evidence argues that SARS-CoV-2 can induce pathologic changes in the brains of several hosts, including humans, although the detailed mechanisms of those pathologies are still elusive. We analyzed the histopathologic changes in the brains on the basis of those uncertain arguments. Of note, we observed drastic pathologic changes in the dog brains, although the animals did not exhibit any neurologic signs. One of the most prominent features of brain pathology was the BBB damage observed during early infection and maintained until later infection. Typical features of vascular damage observed from SVD were changes in the level of tight junction proteins, decreased levels of laminin, and reduced pericyte coverage (27). SVD involves functional and structural dysfunctions of the brain vasculature, demonstrating white matter hyperintensities, microbleeding, and increased perivascular spaces. Moreover, SVD induces increased influx of peripheral blood factors and immune cells into the brain. Our finding of initial pathologic features of the BBB commonly observed in SVD in the brains of SARS-CoV-2–infected dogs strongly supports our hypothesis that the virus can induce SVD in dog brains. Several human studies supporting our observations also reported these pathologic features of the BBB similarly found in SVD (26,28,29).
SVD induces an influx of peripheral molecules and a favorable environment for producing large amounts of reactive oxygen species that activate microglial cells and astrocytes—hallmarks of neuroinflammatory responses. In our study, we specifically observed activation of glial cells in the white matter of the brains of the SARS-CoV-2–infected dogs, suggesting the neuroinflammatory conditions that SARS-CoV-2–mediated SVD might induce. The activation of microglial cells has also been observed in humanized ACE2 mice and brains from different animal species, including nonhuman primates (30,31). The marked axonopathy in the white matter and the preferentially increased activity of glial cells in this region strongly suggest correlations between the glial activation and development of axonopathy potentially mediated by development of SVD by SARS-CoV-2 infection. Furthermore, activation of the astrocytes and microglial cells was maintained up to 40 dpi, even when the virus was cleared from the brain. That finding strongly suggests that the glial cells activated by SARS-CoV-2 potentially harm axons or other components of neuronal cells, even when virus is absent in the brain. That topic could be the focus of future research that requires further in vitro/in vivo studies to reveal the mechanistic link between glial activation and neuronal damage mediated by SARS-CoV-2 infection.
Tau phosphorylation is the hallmark of Alzheimer’s disease. Tau is the family of the microtubule-associated protein tau and functions in the delivery of synaptic vesicles required for synaptic transmission; phosphorylation of tau causes loss of this property, but the mechanism remains elusive (32). There are multiple phosphorylation sites on the tau protein, and our study shows the specific phosphorylation of Ser202/Thr205, detected by using the AT8 antibody (33). Detection of phosphorylated tau suggests a high probability of developing signs of neurodegenerative diseases in the SARS-CoV-2–infected brain. A recent study has shown the correlation between the development of SVD and the accumulation of phosphorylated tau, supporting the finding that development of phosphorylated tau could be oriented by SARS-CoV-2–associated SVD (34,35).
Long-term brain damage induced by SARS-CoV-2 has become a major topic for research of long COVID syndromes in humans (36). It has been reported that ≈10% of SARS-CoV-2–infected persons experience neurologic signs/symptoms, suggesting potential neurotrophic characteristics of this virus (37). According to recent retrospective studies that used UK Biobank data (https://www.ukbiobank.ac.uk), shrinkage in the brain cortex and decreased cognitive function have been reported for human patients after recovery from SARS-CoV-2 infection (38). Moreover, postmortem human brain tissue analysis demonstrated increased activity of glial cells, proinflammatory immune responses, neuronal damage, and BBB damage, enabling peripheral immune cells to infiltrate, strongly suggesting neuropathologic changes induced by SARS-CoV-2 infection (39). However, those pathologic changes were analyzed mainly in brain samples from patients with severe neurologic sign/symptoms; neuropathologic changes in asymptomatic patients are still elusive. From that perspective, our study has value as translational research to predict neuropathologic changes in the early phase of asymptomatic SARS-CoV-2 infection in humans because we have observed the kinetic pathologic changes in the brains of dogs that did not show any neurologic signs. Compared with other animal models, dogs are genetically similar to humans and their brain structures are similar to those of humans, making our extrapolation more reliable. According to our results, the brains of dogs infected with SARS-CoV-2 demonstrate severe BBB disruptions and consequent SVD-like pathologic signs, including axonopathy, glial activation, and potential neurodegenerative changes even without neurologic signs. That evidence strongly suggests that even asymptomatic SARS-CoV-2 patients might have neuropathologic changes in their brains, which could develop into severe neurologic disorders later in life.
Among the merits of our study in terms of translational research of SARS-CoV-2–induced neuropathologic changes, we compared 2 infection routes: direct intranasal infection and horizontal transmission models that can mimic more natural infection routes. With that comparison, we determined that neuropathologic changes can be induced via both exposure routes, providing valuable information that owners of companion animals potentially face SARS-CoV-2–associated neurologic disorders. Second, we studied dogs, which are a more advanced species than rodents, to provide neuropathologic data that are closer to data for humans and more relevant. Moreover, our data suggest that neuropathologic changes can be induced in dogs. Last, we found that the neuroinflammatory responses were more prominently observed in the white matter area than the gray matter area, suggesting that the neuroinflammatory responses induced by SARS-CoV-2 differ by brain region. Overall, these data can be used as translational research data to interpret the potential neuropathologic changes that may be observed in humans.
D.-H. Kim is a PhD candidate at Konkuk University in Seoul. His primary research interests include diagnostics, vaccine development, and antiviral therapeutics, with a particular emphasis on zoonotic viruses.
Acknowledgments
This study was supported by a fund from of Animal and Plant Quarantine Agency, South Korea (project code no. Z-1543085-2022-23-02) and KBRI basic research program through Korea Brain Research Institute funded by the Ministry of Science and ICT (23-BR-04-01 to D.G.K.)
D.-H.K., D.-Y.K., and I.-S.C. conceived and validated the study. Y.-K.S. and O.-K.K. designed the study. D.-H.K., D.-Y.K., K.-S.K., S.-H.H., H.-J.G., J.-H.K., and K.-B.L. contributed to preparing and conducting the experiments. D.-H.L., J.-B.L., S.-Y.P., C.-S.S., S.-W.L., Y.-K.C., and I.-S.C. supervised the experiments. D.-H.K., D.-Y.K., K.-S.K., D.-G.K., and I.-S.C. wrote the main manuscript and prepared the figures. I.-S.C. contributed to funding acquisition. All authors reviewed the final manuscript and approved the submission. All data are presented in the paper and available from the corresponding authors on request.
References
- Abdel-Moneim AS, Abdelwhab EM. Evidence for SARS-CoV-2 infection of animal hosts. Pathogens. 2020;9:529. DOIPubMedGoogle Scholar
- Mahdy MAA, Younis W, Ewaida Z. An overview of SARS-CoV-2 and animal infection. Front Vet Sci. 2020;7:
596391 . DOIPubMedGoogle Scholar - Lee DH, Helal ZH, Kim J, Hunt A, Barbieri A, Tocco N, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a dog in Connecticut in February 2021. Viruses. 2021;13:2141. DOIPubMedGoogle Scholar
- Sit THC, Brackman CJ, Ip SM, Tam KWS, Law PYT, To EMW, et al. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–8. DOIPubMedGoogle Scholar
- Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, et al. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc Natl Acad Sci U S A. 2020;117:26382–8. DOIPubMedGoogle Scholar
- Lyoo KS, Yeo YH, Lee SG, Yeom M, Lee JY, Kim KC, et al. Susceptibility to SARS-CoV-2 and MERS-CoV in beagle dogs. Animals (Basel). 2023;13:624. DOIPubMedGoogle Scholar
- Lyoo KS, Lee H, Lee SG, Yeom M, Lee JY, Kim KC, et al. Experimental infection and transmission of SARS-CoV-2 Delta and Omicron variants among beagle dogs. Emerg Infect Dis. 2023;29:782–5. DOIPubMedGoogle Scholar
- Zhang Z, Zhang Y, Liu K, Li Y, Lu Q, Wang Q, et al. The molecular basis for SARS-CoV-2 binding to dog ACE2. Nat Commun. 2021;12:4195. DOIPubMedGoogle Scholar
- Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371:172–7. DOIPubMedGoogle Scholar
- Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28:2406–15. DOIPubMedGoogle Scholar
- Rass V, Beer R, Schiefecker AJ, Lindner A, Kofler M, Ianosi BA, et al. Neurological outcomes 1 year after COVID-19 diagnosis: A prospective longitudinal cohort study. Eur J Neurol. 2022;29:1685–96. DOIPubMedGoogle Scholar
- Fleischer M, Köhrmann M, Dolff S, Szepanowski F, Schmidt K, Herbstreit F, et al. Observational cohort study of neurological involvement among patients with SARS-CoV-2 infection. Ther Adv Neurol Disord. 2021;14:
1756286421993701 . DOIPubMedGoogle Scholar - Tsivgoulis G, Palaiodimou L, Zand R, Lioutas VA, Krogias C, Katsanos AH, et al. COVID-19 and cerebrovascular diseases: a comprehensive overview. Vol. 13. In: Therapeutic Advances in Neurological Disorders. Newbury Park (CA): SAGE Publications Ltd; 2020.
- Mirfazeli FS, Sarabi-Jamab A, Jahanbakhshi A, Kordi A, Javadnia P, Shariat SV, et al. Neuropsychiatric manifestations of COVID-19 can be clustered in three distinct symptom categories. Sci Rep. 2020;10:20957. DOIPubMedGoogle Scholar
- Shabani Z. Demyelination as a result of an immune response in patients with COVID-19. Vol. 121. In: Acta Neurologica Belgica. Berlin: Springer Science and Business Media Deutschland GmbH; 2021. p. 859–66.
- Moonis G, Filippi CG, Kirsch CFE, Mohan S, Stein EG, Hirsch JA, et al. The spectrum of neuroimaging findings on CT and MRI in adults with COVID-19. AJR Am J Roentgenol. 2021;217:959–74. DOIPubMedGoogle Scholar
- Han C, Duan C, Zhang S, Spiegel B, Shi H, Wang W, et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916–23. DOIPubMedGoogle Scholar
- Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al.; HCA Oral and Craniofacial Biological Network. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. DOIPubMedGoogle Scholar
- Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, et al. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med. 2020;383:592–4. DOIPubMedGoogle Scholar
- Port JR, Yinda CK, Owusu IO, Holbrook M, Fischer R, Bushmaker T, et al. SARS-CoV-2 disease severity and transmission efficiency is increased for airborne compared to fomite exposure in Syrian hamsters. Nat Commun. 2021;12:4985. DOIPubMedGoogle Scholar
- Jonczyk R, Stanislawski N, Seiler LK, Blume H, Heiden S, Lucas H, et al. Combined prospective seroconversion and PCR data of selected cohorts indicate a high rate of subclinical SARS-CoV-2 infections—an open observational study in Lower Saxony, Germany. Microbiol Spectr. 2022;10:
e0151221 . DOIPubMedGoogle Scholar - Patania OM, Chiba S, Halfmann PJ, Hatta M, Maemura T, Bernard KA, et al. Pulmonary lesions induced by SARS-CoV-2 infection in domestic cats. Vet Pathol. 2022;59:696–706. DOIPubMedGoogle Scholar
- Clancy CS, Shaia C, Munster V, de Wit E, Hawman D, Okumura A, et al. Histologic pulmonary lesions of SARS-CoV-2 in 4 nonhuman primate species: An institutional comparative review. Vet Pathol. 2022;59:673–80. DOIPubMedGoogle Scholar
- Kinnare N, Hook JS, Patel PA, Monson NL, Moreland JG. Neutrophil extracellular trap formation potential correlates with lung disease severity in COVID-19 patients. Inflammation. 2022;45:800–11. DOIPubMedGoogle Scholar
- Liu F, Han K, Blair R, Kenst K, Qin Z, Upcin B, et al. SARS-CoV-2 infects endothelial cells in vivo and in vitro. Front Cell Infect Microbiol. 2021;11:
701278 . DOIPubMedGoogle Scholar - Barbosa-Silva MC, Santos LE, Rangel B. The impact of non-neurotropic influenza strains on the brain: a role for microglial priming? J Neurosci. 2018;38:7758–60. DOIPubMedGoogle Scholar
- Li Q, Yang Y, Reis C, Tao T, Li W, Li X, et al. Cerebral small vessel disease. Cell Transplant. 2018;27:1711–22. DOIPubMedGoogle Scholar
- Gyanwali B, Lui B, Tan CS, Chong EJY, Vrooman H, Chen C, et al. Cerebral microbleeds and white matter hyperintensities are associated with cognitive decline in an Asian memory clinic study. Curr Alzheimer Res. 2021;18:399–413. DOIPubMedGoogle Scholar
- Østergaard L, Engedal TS, Moreton F, Hansen MB, Wardlaw JM, Dalkara T, et al. Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab. 2016;36:302–25. DOIPubMedGoogle Scholar
- Rutkai I, Mayer MG, Hellmers LM, Ning B, Huang Z, Monjure CJ, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. 2022;13:1745. DOIPubMedGoogle Scholar
- Seehusen F, Clark JJ, Sharma P, Bentley EG, Kirby A, Subramaniam K, et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. Viruses. 2022;14:1020. DOIPubMedGoogle Scholar
- Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther. 2011;17:514–24. DOIPubMedGoogle Scholar
- Neddens J, Temmel M, Flunkert S, Kerschbaumer B, Hoeller C, Loeffler T, et al. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:52."https://doi.org/10.1186/s40478-018-0557-6" DOIPubMedGoogle Scholar
- Kapasi A, Yu L, Petyuk V, Arfanakis K, Bennett DA, Schneider JA. Association of small vessel disease with tau pathology. Acta Neuropathol. 2022;143:349–62. DOIPubMedGoogle Scholar
- Agrawal S, Yu L, Kapasi A, James BD, Arfanakis K, Barnes LL, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol. 2021;31:
e12939 . DOIPubMedGoogle Scholar - Washington University in St. Louis. COVID-19 infections increase risk of long-term brain problems: strokes, seizures, memory and movement disorders among problems that develop in first year after infection. Science Daily [cited 2023 Apr 10]. https://www.sciencedaily.com/releases/2022/09/220922124408.htm
- Ozel T, Erdem NS, Ünal A, Yalçin AN, İnan D, Ilhanli N, et al. Neurological manifestations and mortality in hospitalized coronavirus disease 2019 patients. Neurological Sciences and Neurophysiology. 2022;39:138–45. DOIGoogle Scholar
- Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. DOIPubMedGoogle Scholar
- Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–29. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: October 13, 2023
1These authors contributed equally to this article.
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
In-Soo Choi, Konkuk University, Veterinary Infectious Diseases, #1 Hwayang-dong Gwanjin-gu, Seoul 143-701, South Korea
Top