Volume 29, Number 5—May 2023
Research
Leishmania donovani Transmission Cycle Associated with Human Infection, Phlebotomus alexandri Sand Flies, and Hare Blood Meals, Israel1
Cite This Article
Citation for Media
Abstract
Cutaneous leishmaniasis caused by Leishmania major or L. tropica and visceral leishmaniasis caused by L. infantum have been reported in Israel. We collected Phlebotomus spp. sand flies in the Negev desert of southern Israel to identify circulating Leishmania spp. Of 22,636 trapped sand flies, 80% were P. alexandri. We sequenced Leishmania-specific internal transcribed spacer 1 fragments and K26 genes. Of 5,019 Phlebotomus female sand flies, 2.5% were Leishmania DNA–positive; 92% of infections were L. donovani. Phylogenetic analyses showed separate clustering of L. donovani and L. infantum. P. alexandri flies positive for L. donovani harbored blood meals from European hares. Leishmania DNA isolated from a patient with cutaneous leishmaniasis who lived in the survey area was identical to L. donovani from P. alexandri flies. We report circulation of L. donovani, a cause of visceral leishmaniasis, in southern Israel. Prompt diagnosis and Leishmania spp. identification are critical to prevent leishmaniasis progression.
Zoonotic leishmaniasis is endemic to Israel. Leishmania tropica, L. major, and L. infantum infect humans in different areas of Israel and circulate through distinct zoonotic transmission cycles (1). Cutaneous leishmaniasis (CL) is caused by L. major, which is transmitted by Phlebotomus papatasi sand flies, and L. tropica, which is transmitted by P. sergenti and P. arabicus sand flies. Canine leishmaniasis and human visceral leishmaniasis (VL) are caused by L. infantum in Israel, and the putative vectors are P. perfiliewi, P. syriacus, and P. tobbi sand flies (1–9). Reservoirs for L. major are sand rats (Psammomys obesus), gerbils (Gerbillus dasyurus), jirds (Meriones crassus and M. tristrami), and possibly also voles (10–13), whereas rock hyraxes (Procavia capensis) are considered the animal reservoir for L. tropica in Israel (14). Domestic dogs (Canis lupus familiaris), jackals (C. aureus), foxes (Vulpes vulpes), and wolves (C. lupus) are recognized reservoir hosts for L. infantum (15).
A substantial increase in CL incidence has been recorded since 2002, and endemic transmission has occurred in areas of Israel where it was previously unknown (6,16,17). Although not life-threatening, CL is a considerable public health problem in Israel; CL is diagnosed in hundreds of new patients annually. During 2001–2018, CL incidence rates increased 7-fold, from 0.4 to 2.9/100,000 population; a peak was observed in 2012, when the mean annual incidence increased to 4.4/100,000 population (18,19). Our study combines results from sand fly surveys, Phlebotomus spp. blood meal analysis, and human patient clinical data from the mountainous area of central Negev in southern Israel during the summer months of 2018–2020. We found a fourth leishmaniasis transmission cycle associated with human illness.
Study Area and Sand Fly Trapping
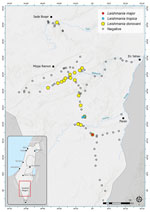
We conducted our study in the mountainous desert area of central Negev in southern Israel (Figure 1). In this region, elevations range from 50 to 1,037 m above sea level, large differences occur between peak daytime and nighttime temperatures, and annual average precipitation is 30–150 mm (20,21). We collected sand flies outdoors in August 2018, September 2019, and August 2020 by using modified traps from the US Centers for Disease Control and Prevention. The traps operated without light and were powered by 2 AA (1.2V) rechargeable batteries and baited with ≈1 kg dry ice. We placed traps in an updraft vertical position overnight; openings were ≈10 cm above the ground, and collection cups hung above the motor and fan (22,23).
Identification and Sample Preparation
We transferred live sand fly catches to the laboratory, which we then chilled and processed. We counted dead sand flies and sorted by sex, identifying all male flies at the species level by using specific morphologic keys for genitalia (24,25). We kept all engorged females and <10–15 unfed females from each trap individually. If the number of female sand flies in the trap was >15, we pooled those flies with others in groups of 20 specimens each. We noted the blood meal size and freshness for each engorged female (26). We stored all female fly specimens in collection microtubes at −80°C until DNA extraction.
Molecular Analysis by Real-Time PCR, HRM Assay, and Sequencing
We extracted total DNA from sand fly samples by using the QIAsymphony DSP DNA Mini Kit and QIAsymphony SP robot (QIAGEN, https://www.qiagen.com). We homogenized the samples for 5 min in 50 μL lysis buffer and stainless steel beads by using a TissueLyser II instrument (QIAGEN). The lysis buffer contained DNase- and proteinase-free RNaseA (ThermoFisher Scientific, https://www.thermofisher.com), proteinase K, and ATL tissue lysis buffer (QIAGEN). After homogenization, we added 200 μL lysis buffer to each samples and incubated at 56°C for 2 h. We performed centrifugation and transferred the samples directly to the robot. We extracted DNA in accordance with the manufacturer’s instructions and eluted the DNA in 100 μL of elution buffer.
We performed all real-time PCR reactions by using a Roche LightCycler 96 (Roche, https://www.roche.com) and AccuMelt HRM SuperMix (Quantabio, https://www.quantabio.com). We analyzed all female sand flies for Leishmania spp. infection and single and engorged female flies to determine Phlebotomus sand fly species and blood meal source. We performed high-resolution melting (HRM) assays at the final step of each real-time PCR. We performed amplicon dissociation analysis by capturing fluorescence signals in 0.1°C/s increments and holding for 60 s in each range of the melting curve (60°C–85°C for sand fly species and blood meal detection assays or <95°C for Leishmania PCR). Sanger sequencing was performed at the Center for Genomic Technologies at Hebrew University of Jerusalem.
We screened all female sand flies for Leishmania DNA and identified parasite species by amplifying an internal transcribed spacer (ITS) 1 rRNA fragment with ITS1–219 PCR primers (Appendix Table 1) and by using the HRM assay (27). For PCR controls, we extracted DNA from parasite promastigote cultures of international reference strains: L. major (MHOM/PS/1967/JerichoII), L. tropica (MHOM/IL/1990/P283), L. infantum (MHOM/SD/62/2S), L. donovani (MHOM/SD/1962/1S-CLD2), and L. aethiopica (MHOM/ET/1972/L102). High purity water for molecular biology (Bio-Lab, http://www.biolab-chemicals.com) was used as a negative control.
We included DNA isolated from skin lesions from 4 patients who had leishmaniasis diagnosed at the parasitology laboratory at Soroka Medical Center, Beer-Sheba, Israel; leishmaniasis was caused by L. donovani/L. infantum complex in those patients (Table 1). Leishmaniasis was diagnosed at the hospital by using multiplex real-time PCR with 5 probes for the ITS region of Leishmania sp. (28,29). We analyzed the samples further at the Ministry of Health by using real-time PCR–HRM amplification of the ITS1 fragment and ITS region and then sequencing.
We amplified the entire 1,020-bp ribosomal ITS region from Leishmania-positive field, clinical, and control samples by using PCR primers LITSR and LITSV (Appendix Table 1). If the entire ITS region was not successfully amplified with LITSR and LITSV primers, we used an internal pair of primers, L5.8S and L5.8SR, to amplify ITS1 and ITS2 separately (30). We performed ITS1 amplicon sequencing for 12 of the positive samples, and the entire ITS region was sequenced from 6 unfed and 3 engorged females, 3 pooled Phlebotomus spp. samples, all 4 human samples, and 4 Leishmania-positive controls. We amplified the repeat region of the L. donovani and L. infantum HASPB (known as K26) gene for additional separation of L. donovani complex–positive samples by using primers K26F and K26R (31).
To identify Phlebotomus spp., we amplified a 368–393-bp fragment of the cytochrome b gene by using a universal primer set designed for this study (cytb-F and cytb-R; Appendix Table 1). The specificity of the designed primers was tested against DNA sequences from hematophagous arthropods, including sand flies, mosquitoes, and ticks. Male sand flies identified at the species level by using morphologic characteristics were used as positive controls and molecular biology grade water was used as a negative control. We analyzed all individual samples, and 1 third of samples from each melting curve pattern were sequenced.
We identified blood meal sources in Phlebotomus sand fly specimens by amplifying a 500-bp segment of host 12S and 16S mitochondrial rRNA genes by using modified vertebrate universal primers N12–16F and N12–16R (32). We included negative (water) and positive (100 ng of human DNA) controls in each PCR. We sequenced 50 samples that represented all HRM curve patterns and all female sand flies containing blood meals that had a melting curve of a rare host (<5 samples).
We used DNA from Leishmania reference strains, male sand fly specimens identified by morphologic characteristics, and human blood as templates for real-time PCR and HRM curve standardization. Each species produced a unique melting curve that was easily distinguishable from other species and consistent with observed nucleotide differences (Appendix Figures 1–3). We compared normalized HRM curves of field samples with the positive control included in each PCR, which enabled species determination (27). We validated species identification by sequencing 1 third of the samples; complete matches were observed for speciation by HRM curve analysis and DNA sequencing.
We aligned and corrected nucleotide sequences by using BioNumerics version 8.0 software (Applied Maths, https://www.applied-maths.com) and compared sequences against the GenBank database by using BLASTN (http://blast.ncbi.nlm.nih.gov). We identified Leishmania spp., blood meal sources, and sand fly species on the basis of >98% identity with sequences obtained during the BLAST search. We submitted sequences of the ITS1 fragments and entire ITS and K26 regions obtained in this study to GenBank (Appendix Table 2).
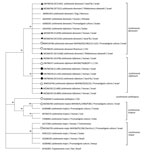
We constructed phylogenetic trees on the basis of marker gene sequences in this study and relevant sequences of other Leishmania spp. deposited in GenBank. We used MEGA X software (33) to infer phylogenetic trees after nucleotide sequence alignment was performed by using ClustalW software (http://www.clustal.org) and maximum-likelihood and neighbor-joining algorithms. We used 1,000 bootstrap replicates to determine percentages of replicate trees. We constructed a phylogenetic tree composed of 45 analyzed partial sequences of the ITS1 locus, including sequences of Leishmania spp. from Israel and other countries deposited in GenBank and Trypanosoma cruzi as an outgroup (Figure 2). We constructed a second tree that included 30 nearly complete ITS sequences of all relevant Leishmania spp. and T. cruzi as an outgroup (Figure 3) and an additional phylogenetic tree that included 20 K26 gene sequences of L. donovani complex–positive samples (Figure 4).
We collected 22,636 Phlebotomus spp. sand fly specimens (15,720 female and 6,916 male; sex ratio 2.3) during 7 trapping nights by using 118 traps placed at 94 sites. After identifying all male and 894 (6%) female flies, we found the catches consisted of 5 species. The most abundant sand fly species were P. alexandri (80%), P. kazeruni (14.4%), P. sergenti (3.1%), P. papatasi (2.2%), and P. syriacus (0.3%) (Table 2).
Among the 4,140 unfed female sand flies tested in 210 pools,we found 41 pools were positive for Leishmania spp. In addition, 6/688 single female flies and 4/206 engorged female flies were positive for Leishmania spp. Of the 51 Leishmania-positive samples, the HRM curves of 47 (36 pools, 6 single females, and 3 engorged female flies) were similar to the HRM curve of the L. donovani control (Table 3; Appendix Figure 1). The HRM curves for 2 pooled fly samples from 2018 were identical to the HRM curve of the L. tropica control. One pooled fly sample and 1 engorged female fly collected in 2020 had an HRM curve identical to the L. major control. The ITS1–PCR sequences of 20 samples (11 pools and all 9 single and engorged females) that had HRM curves similar to the L. donovani control HRM curve were also 100% identical to the L. donovani control sequence.
Leishmaniasis was diagnosed in 4 human patients (Table 1). The ITS1 HRM curve and sequence from patient 4 with CL were similar to the L. donovani control and 47 L. donovani–positive Phlebotomus spp. samples. The ITS1 HRM and sequences from patients 1 and 2 with VL and patient 3 with CL were similar to the L. infantum control. Alignment of ITS1 sequences from L. infantum and L. donovani controls, 3 representative sand fly samples showing HRM identical to L. donovani, and the 4 patient samples showed clustering into 2 distinct groups. The first group comprised the L. donovani control, 3 sand fly samples, and patient 4. The second group comprised the L. infantum control and samples from patients 1, 2, and 3. The difference between the groups was at position 71–74 in ITS1; the L. donovani group had a 4-nt (ATAT) insertion that was missing in the L. infantum–positive samples. A comparison of ITS1 sequences with those in GenBank showed 100% query coverage and 99.65%–100% identity with GenBank sequences for L. infantum and L. donovani from various countries (data not shown).
We aligned DNA sequences from the entire ITS region obtained from Leishmania–positive P. alexandri samples from our study and the L. infantum and L. donovani controls (Appendix Figure 4). We found 2 additional regions containing polymorphic sites that distinguished between L. infantum and L. donovani: a 2-nt (GG) deletion at position 724–725 and 1-nt (G) insertion at position 817 in the L. donovani sequence.
We constructed a phylogenetic tree of ITS1 rRNA fragments of Leishmania sequences obtained from P. alexandri flies, pooled female Phlebotomus spp. flies, and patient samples from this study. We compared those sequences with Leishmania spp. controls and GenBank sequences from Israel and other countries. The tree showed substantial separate clustering of L. infantum (boostrap 94%) and L. donovani (bootstrap 89%) sequences (Figure 2). Phylogenetic analysis of the entire ITS region showed separate clustering of L. infantum (bootstrap 84%) and L. donovani (bootstrap 95%) sequences (Figure 3). K26 phylogenetic analysis also showed separation between L. infantum and L. donovani (Figure 4).
We identified the blood meal source for 182/206 (88%) engorged female sand flies that represented the 4 most abundant sand fly species within the study area. We observed 7 types of HRM curves. We compared blood meal sequences with GenBank sequences and determined similarities between HRM curves. We identified European brown hare (Lepus europaeus) blood in 126 (69.2%) flies, onager (Equus hemionus) blood in 33 (18.3%) flies, gazelle (Gazella dorcas) blood in 16 (8.8%) flies, and domestic dog (C. lupus familiaris) blood in 4 (2.2%) flies; 1 female sand fly each contained blood from either a fat sand rat (Psammomys obesus), fox (V. vulpes), or human (Table 4). Hare blood was the dominant blood meal found in all 4 Phlebotomus spp. flies: P. papatasi, 38%; P. sergenti, 67%; P. alexandri, 71%; and P. kazeruni, 89%. The 12S–16S hare blood meal sequences were 99.8% similar to L. europaeus hares and only 95.3% similar to L. capensis hares.
Of the 47 Phlebotomus spp. sand fly samples with HRM curve patterns and sequences similar to the L. donovani control, 9 were single P. alexandri female sand flies, 3 of which were engorged with hare blood. Of the 2 Phlebotomus spp. samples positive for L. major, 1 was in a single engorged P. papatasi female sand fly that had an unsuccessful blood meal identification. The 2 identified L. tropica samples were from pooled female sand flies.
We found a fourth transmission cycle of leishmaniasis in the central Negev region of southern Israel. On the basis of molecular analysis of the ITS region and K26 gene and phylogenetic analysis, we concluded that the parasite found in patient 4, who lives in the survey area, and in P. alexandri sand flies was L. donovani sensu stricto. We found that L. infantum was the cause of illness in the other 3 patients with leishmaniasis. A case report describing patient 4 was published in 2016; the authors concluded that the infecting parasite was likely L. infantum because of prevailing knowledge of endemic Leishmania transmission in Israel (29). An earlier study reported another patient from the Arava region of central Negev, close to where patient 4 lives, who had symptoms of both CL and VL (34). The cause of infection was identified as L. donovani; the authors noted that this infection was unusual because L. donovani was not known to circulate in Israel. The earlier study substantiates our findings of L. donovani in both sand flies and another human patient within the same geographic area. L. infantum was identified as the causative agent in the other 3 patients in our study and was also described in canines in Israel (1).
The high abundance of P. alexandri sand flies within the study area and the association with L. donovani infections suggest that the P. alexandri sand fly is the putative vector of L. donovani in Israel. P. alexandri flies have been associated with L. donovani sensu latu transmission in other parts of the Old World. Natural infection by L. donovani was found in field-collected P. alexandri sand fly specimens in China, and inoculation of hamsters with those parasites caused VL (35). Another study reported the susceptibility of P. alexandri to artificial infection with L. donovani isolated from human patients in China (36).
We found blood meals from European brown hares in ≈70% of engorged female Phlebotomus sand flies in our study. The high feeding rates on hares, presence of L. donovani in female P. alexandri sand flies engorged with hare blood and illnesses reported in humans infected with L. donovani suggest a zoonotic L. donovani transmission cycle in Israel. Those data suggest that the hare could be a potential reservoir and P. alexandri flies could be the putative vector for L. donovani. The role of hares as a reservoir host for L. donovani requires further investigation; however, a related hare species, Lepus granatensis, was reported as a potential sylvatic reservoir for L. infantum in a leishmaniasis outbreak in Madrid, Spain (37,38). Furthermore, studies in Greece and Italy detected L. donovani complex infection in L. europaeus hares (39,40), providing support for hares as a potential reservoir for L. donovani in Israel. Dogs were identified as reservoirs for L. donovani in India, Sudan, and Ethiopia, and different rodent species have been identified as possible reservoirs of Leishmania spp. from the L. donovani complex (41–48). However, no L. donovani infections in canines and rodents have been reported in Israel; infections in sand fly blood meals found in our study do not implicate those hosts in the local life cycle of L. donovani.
In conclusion, we found circulation of L. donovani in the Negev region of southern Israel that was associated with cutaneous lesions in humans. We determined that P. alexandri was the putative sand fly vector and that hares were the main reservoir host of L. donovani. We found 2 distinct Leishmania spp. in the L. donovani complex in Israel. Previously, the few reported human cases of CL resulting from L. donovani infections were attributed to either L. infantum or nonautochthonous infections. Analysis of patient samples in our study indicates that, in addition to L. major and L. tropica (the known agents causing CL), L. donovani is also a cause of autochthonous CL in Israel. Our results suggest that CL in Israel can be caused by L. donovani, a primary cause of VL. Therefore, prompt diagnosis, identification of the Leishmania sp., and treatment with drugs intended for visceral leishmaniasis, such as pentavalent antimonials or liposomal amphotericin B (49), are critical to prevent disease progression and death among patients with leishmaniasis.
Ms. Studentsky is a PhD student at the Koret School of Veterinary Medicine, The Hebrew University of Jerusalem, Israel, and works at the Medical Entomology Laboratory, Ministry of Health, Jerusalem. Her primary research interests focus on leishmaniasis, sand fly surveillance, and vector competence.
Acknowledgments
We thank Simha Shilo, Anna Sarner, and Arik Levy for assisting with sand fly sorting; Aviv Rashti, Anat Berkowitz, Karin Cohen, and Alina Bazarski for assisting with human sample analysis; Itay Naveh and Dan Ish-Shalom for supporting and participating in the fieldwork; Heather Schnur for editorial remarks; Yaarit Nachum-Biala for sharing professional knowledge of phylogenetic tree construction; and Guy Nizry for helping with map creation.
This study was supported by internal funding from the Ministry of Health, Jerusalem, Israel.
References
- Jaffe CL, Baneth G, Abdeen ZA, Schlein Y, Warburg A. Leishmaniasis in Israel and the Palestinian Authority. Trends Parasitol. 2004;20:328–32. DOIPubMedGoogle Scholar
- Schlein Y, Warburg A, Schnur LF, Gunders AE. Leishmaniasis in the Jordan Valley II. Sandflies and transmission in the central endemic area. Trans R Soc Trop Med Hyg. 1982;76:582–6. DOIPubMedGoogle Scholar
- Schlein Y, Warburg A, Schnur LF, Le Blancq SM, Gunders AE. Leishmaniasis in Israel: reservoir hosts, sandfly vectors and leishmanial strains in the Negev, Central Arava and along the Dead Sea. Trans R Soc Trop Med Hyg. 1984;78:480–4. DOIPubMedGoogle Scholar
- Anis E, Leventhal A, Elkana Y, Wilamowski A, Pener H. Cutaneous leishmaniasis in Israel in the era of changing environment. Public Health Rev. 2001;29:37–47.PubMedGoogle Scholar
- Jacobson RL, Eisenberger CL, Svobodova M, Baneth G, Sztern J, Carvalho J, et al. Outbreak of cutaneous leishmaniasis in northern Israel. J Infect Dis. 2003;188:1065–73. DOIPubMedGoogle Scholar
- Schnur LF, Nasereddin A, Eisenberger CL, Jaffe CL, El Fari M, Azmi K, et al. Multifarious characterization of leishmania tropica from a Judean desert focus, exposing intraspecific diversity and incriminating phlebotomus sergenti as its vector. Am J Trop Med Hyg. 2004;70:364–72. DOIPubMedGoogle Scholar
- Svobodova M, Votypka J, Peckova J, Dvorak V, Nasereddin A, Baneth G, et al. Distinct transmission cycles of Leishmania tropica in 2 adjacent foci, Northern Israel. Emerg Infect Dis. 2006;12:1860–8. DOIPubMedGoogle Scholar
- Jacobson RL. Leishmaniasis in an era of conflict in the Middle East. Vector Borne Zoonotic Dis. 2011;11:247–58. DOIPubMedGoogle Scholar
- Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–50. DOIPubMedGoogle Scholar
- Gunders AE, Foner A, Montilio B. Identification of Leishmania species isolated from rodents in Israel. Nature. 1968;219:85–6. DOIPubMedGoogle Scholar
- Gunders AE, Lidror R, Montilo B, Amitai P. Isolation of Leishmania sp. from Psammomys obesus in Judea. Trans R Soc Trop Med Hyg. 1968;62:465.
- Wasserberg G, Abramsky Z, Anders G, El-Fari M, Schoenian G, Schnur L, et al. The ecology of cutaneous leishmaniasis in Nizzana, Israel: infection patterns in the reservoir host, and epidemiological implications. Int J Parasitol. 2002;32:133–43. DOIPubMedGoogle Scholar
- Faiman R, Abbasi I, Jaffe C, Motro Y, Nasereddin A, Schnur LF, et al. A newly emerged cutaneous leishmaniasis focus in northern Israel and two new reservoir hosts of Leishmania major. PLoS Negl Trop Dis. 2013;7:
e2058 . DOIPubMedGoogle Scholar - Talmi-Frank D, Jaffe CL, Nasereddin A, Warburg A, King R, Svobodova M, et al. Leishmania tropica in rock hyraxes (Procavia capensis) in a focus of human cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;82:814–8. DOIPubMedGoogle Scholar
- Ya’ari A, Jaffe CL, Garty BZ. Visceral leishmaniasis in Israel, 1960-2000. Isr Med Assoc J. 2004;6:205–8.PubMedGoogle Scholar
- Singer SR, Abramson N, Shoob H, Zaken O, Zentner G, Stein-Zamir C. Ecoepidemiology of cutaneous leishmaniasis outbreak, Israel. Emerg Infect Dis. 2008;14:1424–6. DOIPubMedGoogle Scholar
- Azmi K, Krayter L, Nasereddin A, Ereqat S, Schnur LF, Al-Jawabreh A, et al. Increased prevalence of human cutaneous leishmaniasis in Israel and the Palestinian Authority caused by the recent emergence of a population of genetically similar strains of Leishmania tropica. Infect Genet Evol. 2017;50:102–9. DOIPubMedGoogle Scholar
- Gandacu D, Glazer Y, Anis E, Karakis I, Warshavsky B, Slater P, et al. Resurgence of cutaneous leishmaniasis in Israel, 2001-2012. Emerg Infect Dis. 2014;20:1605–11. DOIPubMedGoogle Scholar
- Israel Ministry of Health. Annual report of central laboratories, 2019 (Hebrew) [cited 2021 Jan 5]. https://www.health.gov.il/PublicationsFiles/LAB_JER2019.pdf
- Nezer O, Bar-David S, Gueta T, Carmel Y. High-resolution species-distribution model based on systematic sampling and indirect observations. Biodivers Conserv. 2017;26:421–37. DOIGoogle Scholar
- Stern E, Gardus Y, Meir A, Krakover S, Tzoar H. Atlas of the Negev. Jerusalem (Israel): Keter Publishing House; 1986.
- Orshan L, Szekely D, Khalfa Z, Bitton S. Distribution and seasonality of Phlebotomus sand flies in cutaneous leishmaniasis foci, Judean Desert, Israel. J Med Entomol. 2010;47:319–28. DOIPubMedGoogle Scholar
- Orshan L, Elbaz S, Ben-Ari Y, Akad F, Afik O, Ben-Avi I, et al. Distribution and dispersal of Phlebotomus papatasi (Diptera: Psychodidae) in a zoonotic cutaneous leishmaniasis focus, the northern Negev, Israel. PLoS Negl Trop Dis. 2016;10:
e0004819 . DOIPubMedGoogle Scholar - Abonnenc E. Les Phlébotomes de la région éthiopienne (Diptera, Psychodidae). In: Memoires ORSTOM series. Paris: Office de la Recherche Scientifique et Technique; 1972
- Lewis DJ. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae). Bull Br Mus Nat Hist. 1982;45:121–209.
- Lukes J, Mauricio IL, Schönian G, Dujardin JC, Soteriadou K, Dedet JP, et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc Natl Acad Sci U S A. 2007;104:9375–80. DOIPubMedGoogle Scholar
- Talmi-Frank D, Nasereddin A, Schnur LF, Schönian G, Töz SO, Jaffe CL, et al. Detection and identification of old world Leishmania by high resolution melt analysis. PLoS Negl Trop Dis. 2010;4:
e581 . DOIPubMedGoogle Scholar - Sagi O, Berkowitz A, Codish S, Novack V, Rashti A, Akad F, et al. Sensitive molecular diagnostics for cutaneous leishmaniasis. Open Forum Infect Dis. 2017;4:
ofx037 . DOIPubMedGoogle Scholar - Ben-Shimol S, Sagi O, Horev A, Avni YS, Ziv M, Riesenberg K. Cutaneous leishmaniasis caused by Leishmania infantum in Southern Israel. Acta Parasitol. 2016;61:855–8. DOIPubMedGoogle Scholar
- el Tai NO, Osman OF, el Fari M, Presber W, Schönian G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 2000;94:575–9. DOIPubMedGoogle Scholar
- Haralambous C, Antoniou M, Pratlong F, Dedet JP, Soteriadou K. Development of a molecular assay specific for the Leishmania donovani complex that discriminates L. donovani/Leishmania infantum zymodemes: a useful tool for typing MON-1. Diagn Microbiol Infect Dis. 2008;60:33–42. DOIPubMedGoogle Scholar
- Valinsky L, Ettinger G, Bar-Gal GK, Orshan L. Molecular identification of bloodmeals from sand flies and mosquitoes collected in Israel. J Med Entomol. 2014;51:678–85. DOIPubMedGoogle Scholar
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. DOIPubMedGoogle Scholar
- Ben-Ami R, Schnur LF, Golan Y, Jaffe CL, Mardi T, Zeltser D. Cutaneous involvement in a rare case of adult visceral leishmaniasis acquired in Israel. J Infect. 2002;44:181–4. DOIPubMedGoogle Scholar
- Guan LR, Xu YX, Li BS, Dong J. The role of Phlebotomus alexandri Sinton, 1928 in the transmission of kala-azar. Bull World Health Organ. 1986;64:107–12.PubMedGoogle Scholar
- Guan LR, Zhou ZB, Jin CF, Fu Q, Chai JJ. Phlebotomine sand flies (Diptera: Psychodidae) transmitting visceral leishmaniasis and their geographical distribution in China: a review. Infect Dis Poverty. 2016;5:15. DOIPubMedGoogle Scholar
- Molina R, Jiménez MI, Cruz I, Iriso A, Martín-Martín I, Sevillano O, et al. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet Parasitol. 2012;190:268–71. DOIPubMedGoogle Scholar
- Sevá ADP, Martcheva M, Tuncer N, Fontana I, Carrillo E, Moreno J, et al. Efficacies of prevention and control measures applied during an outbreak in Southwest Madrid, Spain. PLoS One. 2017;12:
e0186372 . DOIPubMedGoogle Scholar - Tsokana CN, Sokos C, Giannakopoulos A, Mamuris Z, Birtsas P, Papaspyropoulos K, et al. First evidence of Leishmania infection in European brown hare (Lepus europaeus) in Greece: GIS analysis and phylogenetic position within the Leishmania spp. Parasitol Res. 2016;115:313–21. DOIPubMedGoogle Scholar
- Rocchigiani G, Ebani VV, Nardoni S, Bertelloni F, Bascherini A, Leoni A, et al. Molecular survey on the occurrence of arthropod-borne pathogens in wild brown hares (Lepus europaeus) from Central Italy. Infect Genet Evol. 2018;59:142–7. DOIPubMedGoogle Scholar
- Jambulingam P, Pradeep Kumar N, Nandakumar S, Paily KP, Srinivasan R. Domestic dogs as reservoir hosts for Leishmania donovani in the southernmost Western Ghats in India. Acta Trop. 2017;171:64–7. DOIPubMedGoogle Scholar
- Hassan MM, Osman OF, El-Raba’a FM, Schallig HD, Elnaiem DE. Role of the domestic dog as a reservoir host of Leishmania donovani in eastern Sudan. Parasit Vectors. 2009;2:26. DOIPubMedGoogle Scholar
- Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, et al. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103–8. DOIPubMedGoogle Scholar
- Bsrat A, Berhe M, Gadissa E, Taddele H, Tekle Y, Hagos Y, et al. Serological investigation of visceral Leishmania infection in human and its associated risk factors in Welkait District, Western Tigray, Ethiopia. Parasite Epidemiol Control. 2017;3:13–20. DOIPubMedGoogle Scholar
- Bashaye S, Nombela N, Argaw D, Mulugeta A, Herrero M, Nieto J, et al. Risk factors for visceral leishmaniasis in a new epidemic site in Amhara Region, Ethiopia. Am J Trop Med Hyg. 2009;81:34–9. DOIPubMedGoogle Scholar
- Kalayou S, Tadelle H, Bsrat A, Abebe N, Haileselassie M, Schallig HDFH. Serological evidence of Leishmania donovani infection in apparently healthy dogs using direct agglutination test (DAT) and rk39 dipstick tests in Kafta Humera, north-west Ethiopia. Transbound Emerg Dis. 2011;58:255–62. DOIPubMedGoogle Scholar
- Magri A, Galuppi R, Fioravanti M, Caffara M. Survey on the presence of Leishmania sp. in peridomestic rodents from the Emilia-Romagna Region (North-Eastern Italy). Vet Res Commun. 2023;47:291–6. DOIPubMedGoogle Scholar
- Özbilgin A, Çavuş İ, Yıldırım A, Gündüz C. [Do the rodents have a role in transmission of cutaneous leishmaniasis in Turkey?] [in Turkish]. Mikrobiyol Bul. 2018;52:259–72.PubMedGoogle Scholar
- Frézard F, Aguiar MMG, Ferreira LAM, Ramos GS, Santos TT, Borges GSM, et al. Liposomal amphotericin B for treatment of leishmaniasis: from the identification of critical physicochemical attributes to the design of effective topical and oral formulations. Pharmaceutics. 2022;15:99. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: April 13, 2023
1Data from this study were presented at the Israeli Society for Parasitology, Protozoology and Tropical Diseases Annual Meeting; March 21, 2022; Kfar Hamaccabiah, Ramat Gan, Israel.
Table of Contents – Volume 29, Number 5—May 2023
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Liora Studentsky, Laboratory of Medical Entomology, Ministry of Health, Elaiv Yaakov 9, Jerusalem 9546208, Israel
Top