Volume 30, Number 2—February 2024
Research
Public Health Impact of Paxlovid as Treatment for COVID-19, United States
Figure 2
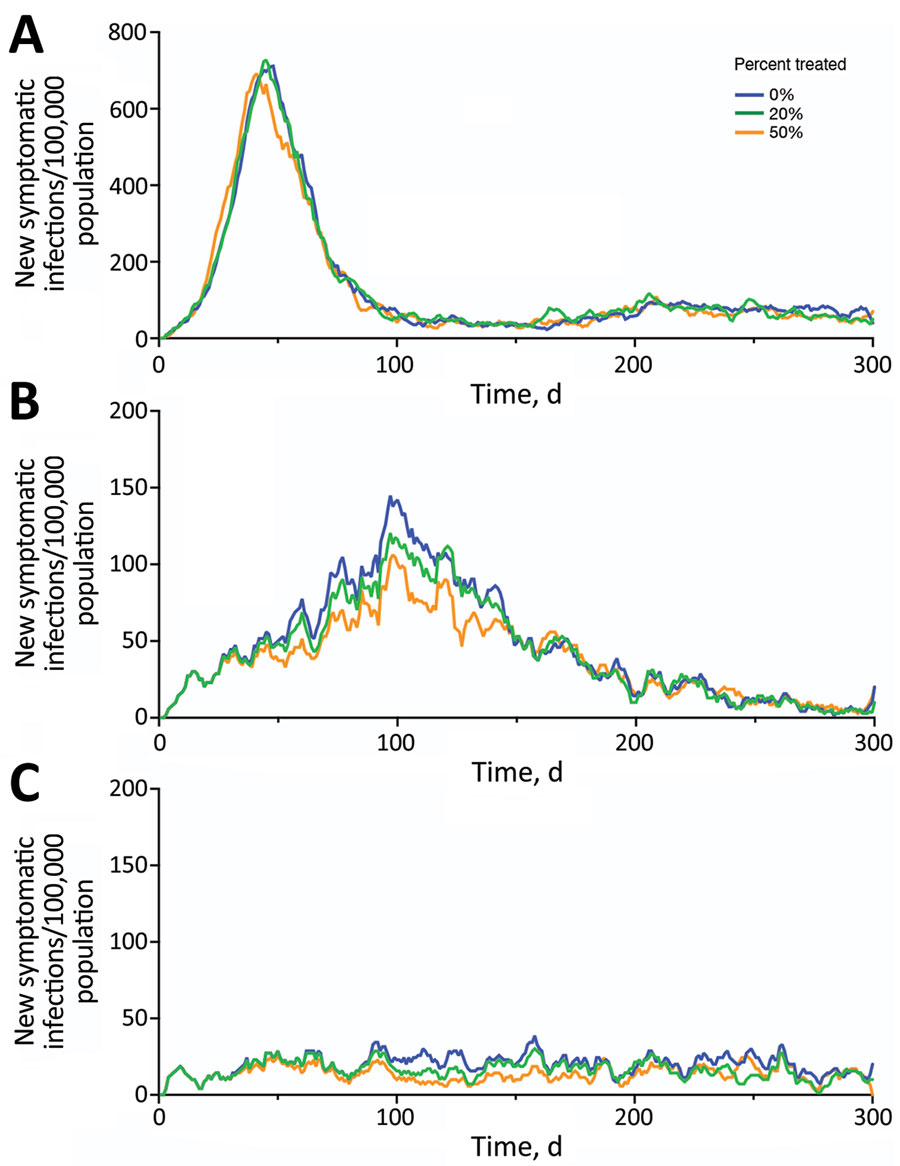
Figure 2. Projected symptomatic SARS-CoV-2 infections over 300 days in the United States across a range of transmission and Paxlovid treatment scenarios. Estimated incidence of symptomatic SARS-CoV-2 infections are shown assuming an effective reproduction number of 3.0 (A), 1.7 (B), or 1.2 (C). Colors correspond to 3 different treatment scenarios: 0% (blue), 20% (green), or 50% (orange) of symptomatic cases received a 5-day Paxlovid regimen initiated within 3 days of symptom onset.
1These authors contributed equally to this article.
Page created: December 21, 2023
Page updated: January 24, 2024
Page reviewed: January 24, 2024
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.