Volume 30, Number 2—February 2024
Research
Public Health Impact of Paxlovid as Treatment for COVID-19, United States
Figure 3
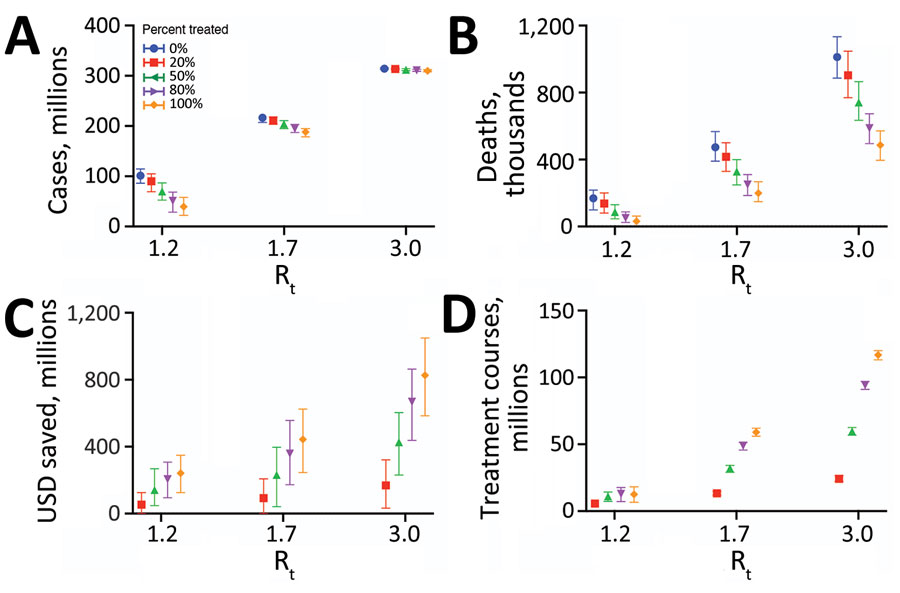
Figure 3. Projected health and economic impacts of a large-scale campaign using Paxlovid to treat COVID-19 over 300 days in the United States, across a range of transmission and treatment scenarios. Points and error bars correspond to means and 95% CI in number of infections in millions (A), number of deaths in millions (B), net monetary benefit in billions USD assuming a treatment course cost of US $530 and willingness to pay per year of life lost averted of US $100,000 (C), and number of courses of Paxlovid administered in millions (D). Each graph provides results for 3 Rt and 5 different treatment scenarios: 0% (blue), 20% (red), 50% (green), 80% (purple), or 100% (orange) of symptomatic cases started a 5-day course of Paxlovid within 3 days of symptom onset. Distributions are based on 100 stochastic simulations for each scenario. The results are scaled assuming a US population of 328.2 million (21). Rt, effective reproduction number; USD, US dollars.
References
- Dadonaite B. Antiretroviral therapy has saved millions of lives from AIDS and could save more [cited 2021 Feb 22]. https://ourworldindata.org/art-lives-saved
- Suda KJ, Hunkler RJ, Matusiak LM, Schumock GT. Influenza antiviral expenditures and outpatient prescriptions in the United States, 2003–2012. Pharmacotherapy. 2015;35:991–7. DOIPubMedGoogle Scholar
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729–37. DOIPubMedGoogle Scholar
- Du Z, Nugent C, Galvani AP, Krug RM, Meyers LA. Modeling mitigation of influenza epidemics by baloxavir. Nat Commun. 2020;11:2750. DOIPubMedGoogle Scholar
- Wahl A, Gralinski LE, Johnson CE, Yao W, Kovarova M, Dinnon KH III, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–7. DOIPubMedGoogle Scholar
- Shah MM, Joyce B, Plumb ID, Sahakian S, Feldstein LR, Barkley E, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19—United States, April–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1531–7. DOIPubMedGoogle Scholar
- Pfizer. Pfizer shares in vitro efficacy of novel COVID-19 oral treatment against Omicron variant [cited 2022 Mar 21]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-vitro-efficacy-novel-covid-19-oral-treatment
- Pfizer. Pfizer to provide U.S. government with an additional 10 million treatment courses of its oral therapy to help combat COVID-19 [cited 2022 Mar 25]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provide-us-government-additional-10-million
- Jenner AL, Aogo RA, Alfonso S, Crowe V, Deng X, Smith AP, et al. COVID-19 virtual patient cohort suggests immune mechanisms driving disease outcomes. PLoS Pathog. 2021;17:
e1009753 . DOIPubMedGoogle Scholar - Kim KS, Ejima K, Iwanami S, Fujita Y, Ohashi H, Koizumi Y, et al. A quantitative model used to compare within-host SARS-CoV-2, MERS-CoV, and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2. PLoS Biol. 2021;19:
e3001128 . DOIPubMedGoogle Scholar - Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al.; EPIC-HR Investigators. EPIC-HR Investigators. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408. DOIPubMedGoogle Scholar
- Handel A, Rohani P. Crossing the scale from within-host infection dynamics to between-host transmission fitness: a discussion of current assumptions and knowledge. Philos Trans R Soc Lond B Biol Sci. 2015;370:
20140302 . DOIPubMedGoogle Scholar - Néant N, Lingas G, Le Hingrat Q, Ghosn J, Engelmann I, Lepiller Q, et al.; French COVID Cohort Investigators and French Cohort Study groups. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci U S A. 2021;118:
e2017962118 . DOIPubMedGoogle Scholar - Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9. DOIPubMedGoogle Scholar
- Du Z, Pandey A, Bai Y, Fitzpatrick MC, Chinazzi M, Pastore Y Piontti A, et al. Comparative cost-effectiveness of SARS-CoV-2 testing strategies in the USA: a modelling study. Lancet Public Health. 2021;6:e184–91. DOIPubMedGoogle Scholar
- U.S. Federal Highway Administration. 2017 national household travel survey [cited 2020 Jun 16]. https://nhts.ornl.gov/downloads
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. DOIPubMedGoogle Scholar
- Czuppon P, Débarre F, Gonçalves A, Tenaillon O, Perelson AS, Guedj J, et al. Success of prophylactic antiviral therapy for SARS-CoV-2: Predicted critical efficacies and impact of different drug-specific mechanisms of action. PLOS Comput Biol. 2021;17:
e1008752 . DOIPubMedGoogle Scholar - Traynard P, Ayral G, Twarogowska M, Chauvin J. Efficient pharmacokinetic modeling workflow with the MonolixSuite: a case study of remifentanil. CPT Pharmacometrics Syst Pharmacol. 2020;9:198–210. DOIPubMedGoogle Scholar
- Lavielle M, Mentré F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J Pharmacokinet Pharmacodyn. 2007;34:229–49. DOIPubMedGoogle Scholar
- US Census Bureau. National population by characteristics: 2010–2019 [cited 2020 Oct 1]. https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html
- Robbins R, Zimmer C. FDA clears Pfizer’s Covid pill for high-risk patients 12 and older. 2021 Dec 22 [cited 2022 Apr 29]. https://www.nytimes.com/2021/12/22/health/pfizer-covid-pill-fda-paxlovid.html
- TIME. China’s approval of Pfizer pill opens door to ending COVID Zero. 2022 Feb 14 [cited 2023 Jan 21]. https://time.com/6147924/china-pfizer-covid-19-pill
- Joshi AU, Lewiss RE, Aini M, Babula B, Henwood PC. Solving community SARS-CoV-2 testing with telehealth: development and implementation for screening, evaluation and testing. JMIR Mhealth Uhealth. 2020;8:
e20419 . DOIPubMedGoogle Scholar - Centers for Disease Control and Prevention. New COVID-19 test to treat initiative and locator tool [cited 2022 Apr 6]. https://emergency.cdc.gov/newsletters/coca/040422.htm
- Huang F, Liu H. The impact of the COVID-19 pandemic and related policy responses on non-COVID-19 healthcare utilization in China. Health Econ. 2023;32:620–38. DOIPubMedGoogle Scholar
- National Health Commission of the People’s Republic of China. China’s internet health services gathering steam amid COVID-19 [cited 2022 Dec 7]. http://en.nhc.gov.cn/2021-08/24/c_85005.htm
- He D, Gu Y, Shi Y, Wang M, Lou Z, Jin C. COVID-19 in China: the role and activities of Internet-based healthcare platforms. Glob Health Med. 2020;2:89–95. DOIPubMedGoogle Scholar
- China Internet Network Information Center. 48th statistical report on internet development in China [cited 2021 Nov 19]. https://www.cnnic.com.cn/IDR/ReportDownloads/202111/P020211119394556095096.pdf
- Vegvari C, Hadjichrysanthou C, Cauët E, Lawrence E, Cori A, de Wolf F, et al. How can viral dynamics models inform endpoint measures in clinical trials of therapies for acute viral infections? PLoS One. 2016;11:
e0158237 . DOIPubMedGoogle Scholar - Iketani S, Mohri H, Culbertson B, Hong SJ, Duan Y, Luck MI, et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature. 2023;613:558–64. DOIPubMedGoogle Scholar
- Callaway E. How months-long COVID infections could seed dangerous new variants. Nature. 2022;606:452–5. DOIPubMedGoogle Scholar
- Herxheimer A. Relationships between the pharmaceutical industry and patients’ organisations. BMJ. 2003;326:1208–10. DOIPubMedGoogle Scholar
- Biggerstaff M, Jhung MA, Reed C, Fry AM, Balluz L, Finelli L. Influenza-like illness, the time to seek healthcare, and influenza antiviral receipt during the 2010-2011 influenza season-United States. J Infect Dis. 2014;210:535–44. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.