Volume 31, Number 2—February 2025
Dispatch
Infection by Tickborne Bacterium Candidatus Midichloria Associated with First Trimester Pregnancy Loss, Tennessee, USA
Cite This Article
Citation for Media
Abstract
A previously healthy 26-year-old woman in middle Tennessee, USA, experienced a first trimester pregnancy loss after multiple tick bites. Histopathology, 16S rRNA sequencing, and electron microscopy examination of the products of conception revealed an infection by a bacterium within the Candidatus Midichloria genus.
Chromosomal aneuploidy accounts for most first trimester pregnancy losses, but infections are estimated to cause ≈15% of early miscarriages (1). Evidence supporting an association of tickborne bacterial infections with early pregnancy loss is scarce and largely limited to case reports and small case series. Tickborne diseases with anecdotal associations to early pregnancy loss include Lyme disease, babesiosis, rickettsial diseases, and ehrlichiosis (2,3).
Candidatus Midichloriaceae represents a family of intracellular bacterial organisms first identified in the ovaries of the Ixodes ricinus tick, the primary vector of Lyme disease in Europe. Phylogenetic analysis led to the proposal for Candidatus Midichloriaceae to be separated into a third family under the order Rickettsiales, distinct from Rickettsiaceae and Anaplasmataceae, but likely more akin to Anaplasmataceae (4). Some members of the family boast a unique intramitochondrial life cycle, demonstrating tropism for a growing number of nonhuman hosts, such as aquatic invertebrates, protists, and various farm animals with unknown pathogenic potential (5). Further, the presence of Candidatus Midichloria genus mitochondrii DNA in human serum has rarely been documented without evidence of pathogenic effects (6). In this study, we describe a case of early pregnancy loss after tick exposure and the subsequent detection of Candidatus Midichloria sp. within the products of conception.
A 26-year-old woman in her first pregnancy sought care at 8 weeks’ gestation at an urgent care center in middle Tennessee, USA, with a 1-day history of a painful and pruritic rash on her torso at the site of a previous tick attachment (Figure 1). The rash consisted of a faint circular area of erythema surrounding a central shallow eschar. Two weeks before the rash developed, the patient removed 4 embedded ticks after walking her dogs in a field. The patient was unable to provide additional information regarding the physical characteristics of the ticks. The patient reported no systemic symptoms or recent domestic or international travel.
The patient was prescribed a 14-day course of cefuroxime axetil. Results of acute and convalescent serologic testing for Ehrlichia chaffeensis, Borrelia burgdorferi, and Rickettsia rickettsii infections were negative (Table). Results of complete blood count were unremarkable. After negative results of serologic testing were received, the patient was instructed to stop the prescribed antibiotic medications after ≈4 days of treatment.
The patient noted vaginal bleeding 2 weeks and 5 days after the urgent care visit; a subsequent prenatal ultrasound confirmed intrauterine fetal demise. The products of conception were routinely submitted for pathologic examination.
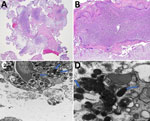
Hematoxylin and eosin–stained sections showed marked acute villitis involving most of the trophoblastic villi. Large intravillous abscesses involved many villi. In addition, the acute inflammatory reaction appeared to originate at the villous tip and spread proximally to involve nearly the entire villous in a confluent fashion (Figure 2, panels A, B). Gram staining for bacteria and immunohistochemistry for cytomegalovirus were both negative. Giemsa special staining (Appendix Figure) showed darkly stained intracellular rods.
Electron microscopy examination of the trophoblastic villi demonstrated intracellular bacterial organisms within cytoplasmic vacuoles (Figure 2, panel C) and freely within the cytosol (Figure 2, panel D). The morphology of the organisms closely resembled that of the species Candidatus Midichloria mitochondrii, although definitive speciation cannot be rendered (7). Specifically, the organisms were round in cross-sectional profiles and appeared as short rods in longitudinal sections, measuring ≈0.25–0.34 µm in diameter and 0.40–0.53 µm in length. The bacteria exhibited an electron dense cytoplasm with vague granular content and an electron lucent outer membrane. Of note, the mitochondria were not involved in the sections examined.
After light microscopy examination, we submitted a formalin-fixed paraffin-embedded tissue block for a clinically available 16S rRNA broad range PCR, followed by Sanger sequencing (8). After primer and quality trimming, the resulting 297-bp product (Genbank accession no. PP102451) shared 95.9% nucleotide identity to Candidatus Midichloria mitochondrii strain IricVA (GenBank accession no. NC_015722) and 95.2% identity to another I. ricinus–associated 16S sequence (GenBank accession no. DQ788562). The identical sequence was subsequently detected in 2 replicate extractions from a second tissue block from the case. To assess for preanalytical contamination, we also tested 2 formalin-fixed paraffin-embedded blocks from different patients processed in the same batch as this case by broad-range bacterial PCR. Both blocks were negative for bacterial DNA, ruling out contamination during tissue processing.
We analyzed the patient-derived 16S sequence in the context of representative Rickettsiales sequences (Figure 3). In the resulting tree, the detected bacterial sequence had the highest homology with a sequence from Thailand (accession no. KY910125) and was in a clade with Candidatus Midichloria spp. (80%–90% bootstrap support). The detected sequence was distinct from Candidatus M. mitochondrii, as well as other endosymbionts within the genus including Candidatus Lariskella sp. (AB624350).
Candidatus Midichloriaceae are proposed as a separate family under the Rickettsiales order, but few studies have attempted to describe the biology of this relatively unexplored region of the bacterial taxonomy. Bacteria of this family have been isolated in numerous tick vectors known to transmit pathogenic bacteria, including members of the Ixodes, Rhipicephalus, Amblyomma, Hyalomma, and Dermacentor genera, and have the capacity for mammalian inoculation (9). Candidatus Midichloriaceae organisms have a predilection for female ticks and demonstrate tropism for the salivary glands and ovaries, enabling vertical transmission to tick progeny (10). Evidence suggests at least facultative mutualism between the tick host and some Candidatus Midichloriaceae organisms; Candidatus M. mitochondrii has been shown to increase the tick’s feeding ability during blood meals, as well as aid in the production of superior larvae (11,12).
The pathogenic potential of Candidatus Midichloriaceae in intermediate hosts is incompletely understood. Three studies have reported isolating antibodies to Ca. Midichloriaceae or microbial DNA from the blood of human hosts exposed to tick bites, with similar detection rates as a unique organism or in combination with another tickborne pathogen (6,13,14). Those results suggest that Candidatus Midichloriaceae antigens or nucleic acids can be transmitted to humans and used as a marker for tick bites but do not demonstrate independent pathogenicity or replicative ability. One of the 3 studies potentially identified a bacterium from the Candidatus Midichloriaceae family causing a mild febrile illness in east Russia in 2004 (14). Our results suggest a strong association between infection by Candidatus Midichloria sp. and the pregnancy loss in this patient.
Although electron microscopy images showed intracellular bacteria that could be compatible with Candidatus Midichloria sp., this study is limited by the lack of clear internal structures, although vague granular content suggestive of organelles was present. Therefore, definitive identification cannot be rendered. The only other known pathogen on the histologic differential diagnosis is Listeria monocytogenes, which classically displays villous microabscesses. However, the abscesses caused by L. monocytogenes are smaller, and the acute inflammation does not tend to be confluent or span the entire length of the villous (15). Further, 16S rRNA sequencing did not detect L. monocytogenes in 2 replicate extractions, and L. monocytogenes organisms are typically situated in phagolysosomes and measure 500–2,000 nm in length.
This case describes an early pregnancy loss associated with an organism within the Candidatus Midichloriaceae family. This finding suggests tropism for human trophoblastic tissue and potentially deleterious effects for the fetus. Although further studies are needed to elucidate the taxonomy, hosts, life cycle, and pathogenesis of Candidatus Midichloria sp. and related endosymbionts, clinicians and patients should be aware of this emerging pathogen. Further, for pregnant patients with tick bites, treatment with doxycycline should be considered because the benefits could outweigh the risks. Pregnant patients, especially those in the southeastern United States, should be counseled on the risk for tick exposure during early pregnancy, as well as on proper tick removal technique.
Dr. Newman is the neuropathology fellow at Stanford University Hospital, California, USA. His primary research interests include neurodegenerative disease, central nervous system neoplasia, and infectious diseases.
References
- Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22:116–33. DOIPubMedGoogle Scholar
- Lambert JS. An overview of tickborne infections in pregnancy and outcomes in the newborn: the need for prospective studies. Front Med (Lausanne). 2020;7:72. DOIPubMedGoogle Scholar
- Weber K, Bratzke HJ, Neubert U, Wilske B, Duray PH. Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatr Infect Dis J. 1988;7:286–9. DOIPubMedGoogle Scholar
- Sassera D, Beninati T, Bandi C, Bouman EAP, Sacchi L, Fabbi M, et al. ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int J Syst Evol Microbiol. 2006;56:2535–40. DOIPubMedGoogle Scholar
- Giannotti D, Boscaro V, Husnik F, Vannini C, Keeling PJ. The “Other” Rickettsiales: an overview of the family “Candidatus Midichloriaceae.” Appl Environ Microbiol. 2022;88:
e0243221 . DOIPubMedGoogle Scholar - Sgroi G, Iatta R, Lovreglio P, Stufano A, Laidoudi Y, Mendoza-Roldan JA, et al. Detection of endosymbiont Candidatus Midichloria mitochondrii and tickborne pathogens in humans exposed to tick bites, Italy. Emerg Infect Dis. 2022;28:1824–32. DOIPubMedGoogle Scholar
- Comandatore F, Radaelli G, Montante S, Sacchi L, Clementi E, Epis S, et al. Modeling the life cycle of the intramitochondrial bacterium “Candidatus Midichloria mitochondrii” using electron microscopy data. MBio. 2021;12:
e0057421 . DOIPubMedGoogle Scholar - McCormick DW, Rassoulian-Barrett SL, Hoogestraat DR, Salipante SJ, SenGupta D, Dietrich EA, et al. Bartonella spp. infections identified by molecular methods, United States. Emerg Infect Dis. 2023;29:467–76. DOIPubMedGoogle Scholar
- Bazzocchi C, Mariconti M, Sassera D, Rinaldi L, Martin E, Cringoli G, et al. Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales: Midichloriaceae) in different mammalian species. Parasit Vectors. 2013;6:350. DOIPubMedGoogle Scholar
- Mukhacheva TA, Kovalev SY. Bacteria of the family ‘Candidatus Midichloriaceae’ in sympatric zones of Ixodes ticks: genetic evidence for vertical transmission. Microb Ecol. 2017;74:185–93. DOIPubMedGoogle Scholar
- Stavru F, Riemer J, Jex A, Sassera D. When bacteria meet mitochondria: The strange case of the tick symbiont Midichloria mitochondrii†. Cell Microbiol. 2020;22:
e13189 . DOIPubMedGoogle Scholar - Guizzo MG, Hatalová T, Frantová H, Zurek L, Kopáček P, Perner J. Ixodes ricinus ticks have a functional association with Midichloria mitochondrii. Front Cell Infect Microbiol. 2023;12:
1081666 . DOIPubMedGoogle Scholar - Mariconti M, Epis S, Gaibani P, Dalla Valle C, Sassera D, Tomao P, et al. Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: is Midichloria a novel pathogen, or just a marker of tick bite? Pathog Glob Health. 2012;106:391–6. DOIPubMedGoogle Scholar
- Mediannikov OI, Ivanov LI, Nishikawa M, Saito R, Sidel’nikov IN, Zdanovskaia NI, et al. [Microorganism “Montezuma” of the order Rickettsiales: the potential causative agent of tick-borne disease in the Far East of Russia] [in Russian]. Zh Mikrobiol Epidemiol Immunobiol. 2004; (
1 ):7–13.PubMedGoogle Scholar - Sepp AH, Roy TE. Listeria monocytogenes infections in Metropolitan Toronto. A clinicopathological study. Can Med Assoc J. 1963;88:549–61.PubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: January 16, 2025
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Hernán Correa, 11219 Doctor’s Office Tower, Vanderbilt Children’s Hospital, 2200 Children’s Way, Nashville, TN 37232-9065, USA
Top