Volume 31, Number 2—February 2025
Research
Prions in Muscles of Cervids with Chronic Wasting Disease, Norway
Cite This Article
Citation for Media
Abstract
Chronic wasting disease (CWD) is an emerging prion disease in Nordic countries and has been detected in reindeer, moose, and red deer since 2016. CWD sporadically detected in moose and red deer in 3 Nordic countries demonstrated pathologic and strain characteristics different from CWD in reindeer, including an unexpected lack of prions outside the central nervous system as measured by standard diagnostic tests. Using protein misfolding cyclic amplification, we detected prions in the lymphoreticular system of moose and red deer with CWD in Norway and, remarkably, in muscles of both of those species and in CWD-infected reindeer. One moose lymph node and 1 moose muscle sample showed infectivity when experimentally transmitted to bank voles. Our findings highlight the systemic nature of CWD strains in Europe and raise questions regarding the risk of human exposure through edible tissues.
Chronic wasting disease (CWD) is a fatal disease affecting several species of cervids (1,2). It belongs to the group of transmissible spongiform encephalopathies, including scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle, and Creutzfeldt-Jakob disease in humans (3). Transmissible spongiform encephalopathies are caused by misfolded forms of the prion protein (PrPC), known as PrPSc (prions), which are thought to replicate in an autocatalytic process that converts PrPC into PrPSc (4). The diseases are characterized by spongiform changes and the accumulation of PrPSc in the central nervous system (CNS).
Researchers first described CWD in North America in 1967, and evidence of the disease has since then inexorably expanded across 34 states in the United States and 4 provinces in Canada (5). CWD emerged for the first time in Europe in a wild reindeer (Rangifer tarandus) in Norway and, shortly thereafter, in 2 moose (Alces alces). As part of an extensive surveillance program in Norway, investigators have identified 21 reindeer, 13 moose, and 3 red deer (Cervus elaphus) as being infected with CWD. A 3-year surveillance program in countries in Europe that have wild reindeer or moose revealed CWD in 3 moose in Finland and in 4 moose in Sweden.
The origin of the CWD disease identified in Europe is not known. Increasing data showed that the prion strains found in cases from northern Europe were multiple and different from those in North America (6–8). The strain found in reindeer closely resembled the North America strains in terms of distribution of PrPSc, first in the lymphoreticular system and later in the brain, and the contagious characteristics in the natural host. Nevertheless, the strain of CWD found in reindeer in Europe was not identical to the CWD characterized from North America (9,10). Furthermore, CWD strains from moose in Nordic countries demonstrated substantial differences when compared with North America strains. Those moose, primarily old animals with a sporadic geographic distribution in Norway, Finland, and Sweden, exhibited unique characteristics not previously documented, and their infections were proposed as sporadic CWD (11). Transmission studies in bank voles and in transgenic mice expressing cervid PrP revealed that CWD prions in moose clearly differ from CWD prions from both reindeer in Norway and from the North America isolates studied. Furthermore, researchers have observed PrPSc and strain variation among individual moose isolates (9,10,12,13). Researchers studying CWD-affected moose and using traditional immunodetection tests (ELISA, Western blot [WB], and immunohistochemistry) detected PrPSc in the CNS and not in the lymphoreticular system (13,14). As in moose, PrPSc is not lymphotropic in red deer, but analysis on the first CWD-affected red deer in Norway revealed numerous characteristics that point to a unique CWD strain (6,15–18).
CWD has an incubation period of several years, during which time infected animals can shed prions in excreta, even before showing clinical signs (19–22). On the basis of investigations of sheep scrapie and cattle BSE, researchers have commonly hypothesized that CWD cases demonstrating a disease phenotype with general lymphoreticular involvement have higher potential to transmit the disease in field conditions (23). The anatomic distribution in the CNS and the lymphotropic properties of PrPSc distinguish the different prion strains (24,25). Those insights have inspired conjecture regarding a possible (albeit undetectable with conventional assays) accumulation of infectious prions in the peripheral tissues of the newly emerged strains in cervids in Norway, especially in the strains identified in moose and red deer. We used protein misfolding cyclic amplification (PMCA) and animal bioassay to assess the presence of CWD prions and their potential infectivity in lymph nodes, nerves, and muscles from CWD field cases in Norway.
Animal Tissues
We detected CWD-affected animals (Table 1) through Norway’s surveillance program for CWD by using TeSeE (Bio-Rad Laboratories, https://www.bio-rad.com) or HerdChek (IDEXX, https://www.idexx.com) ELISA tests and confirmed results by TeSeE Western blot (Bio-Rad). We collected tissue samples from different parts of 2 reindeer, 4 moose, and 1 red deer by using disposable instruments to avoid cross contamination (Table 2). We used 3 reindeer, 3 moose and 1 red deer, all healthy and confirmed PrPSc-negative, as negative controls.
Tissues Preparation
We homogenized all biologic tissues in phosphate-buffered saline at 10% wt/vol and used grinding tubes with silica beads and TeSeE PRECESS 48 homogenizer (Bio-Rad). For the muscle and lymph node tissues, we applied 2 ribolysing cycles of 60 seconds ribolysis and 30 seconds rest to improve the homogenization quality. Except for the brain samples that were tested at 10−4 to 10−8 dilutions to assess PMCA sensitivity, we tested all samples undiluted.
PMCA
Scientists performed PMCA in parallel in laboratories in Ås (Lab-Å) and in Milan (Lab-M). We prepared PMCA substrate by homogenizing bank vole brains (PRNP 109M; obtained from the Italian National Institute of Health, Rome) at 10% wt/vol in conversion buffer consisting of phosphate-buffered saline supplemented with a cocktail of 150 mM NaCl, 1% Triton X-100, and protease inhibitors (Roche, https://www.roche.com). We removed debris by centrifugation at 800 × g for 1 minute before aliquoting and storing the supernatant at −80°C. Before PMCA, we supplemented the substrate with 0.1 mg/mL heparin and 0.05% digitonin to increase the amplification efficiency.
To run PMCA analyses, we added 10 µL of each sample to 90 µL of PMCA reaction substrate in a PCR tube with 3 poly(tetrafluoroethylene) beads (Marteau & Lemarié, https://www.marteau-lemarie.fr). We then subjected the mixtures to cycles of sonication (30 seconds at 230–250 watts) and incubation (29 minutes 30 seconds at 36°C–40°C) using a Q700 sonicator (QSonica, https://www.sonicator.com), in 96 cycles for 1 PMCA round. We employed a slightly different experimental setting in Lab-M: 20 seconds sonication at 150–170 watts and 29 minutes 40 seconds incubation at 37°C. When available, we analyzed equivalent tissues from CWD-negative animals in parallel as negative controls for the amplification. We performed a maximum of 6 PMCA rounds and evaluated the resulting PMCA products by WB.
WB Analysis
Brain Isolates
We prepared and analyzed brain homogenates according to the instructions of the TeSeE Western blot kit (Bio-Rad), with a slight modification: we performed the electrophoresis using NuPAGE 12% Bis-Tris protein gels (Thermo Scientific, https://www.thermofisher.com). To distinguish the PrPres (protease-resistant prion) types according to the differential proteinase K cleavage at the N terminus, we used 2 different antibodies: Sha31 antibody (TeSeE Western blot kit), which recognizes the epitope (aa 145–152) in the core protein; and monoclonal antibody (mAb) 12B2 (1:1000; Wageningen Bioveterinary Research, https://www.wur.nl), which binds the epitope (aa 89–93) situated at N-terminal of the prion protein.
PMCA Products
We performed WB analysis of the PMCA products by using a standard protocol, with slight differences between Lab-Å and Lab-M. We treated the amplified products with proteinase K (Sigma-Aldrich Solutions, https://www.sigmaaldrich.com) at a final concentration of 100 µg/mL (or 50 µg/mL) for 1 hour at 37°C. We halted digestion by adding Laemmli buffer and boiling the samples at 100°C for 5 minutes (or 10 minutes) before conducting sodium dodecyl sulfate–polyacrylamide gel electrophoresis by using NuPAGE 12% Bis-Tris protein gels (Thermo Scientific). We transferred proteins onto a polyvinylidene difluoride membrane and subjected them to immunodetection using the mAb Sha31clone, at a dilution of 1:10 (TeSeE Western blot kit [Bio-Rad]), or the mAb 6D11 clone (Biolegend, https://www.biolegend.com), at a dilution of 1:5000. We developed results by using SuperSignal West Pico plus chemiluminescent substrate (Thermo Scientific) or ECL Prime (GE Healthcare, https://www.gehealthcare.com) and visualized using Azure c280 (Azure Biosystems, https://azurebiosystems.com). We considered a sample positive upon detection of a pattern of 3 protease-resistant bands at expected electrophoretic mobility within 6 rounds of PMCA. We considered samples inconclusive when results differed in 2 different laboratories.
Bioassay
Bank voles carrying isoleucine at the polymorphic PRNP codon 109 (Bv109I) were bred and inoculated at the Istituto Superiore di Sanità after approval of the experimental protocol from the Italian Ministry of Health (decree number 1122/2020-PR). We carried out all procedures in accordance with European Council directive 2010/63 and in compliance with the Italian Legislative Decree 26/2014. We inoculated 8-week-old Bv109I voles intracerebrally with 20 μL of 10% wt/vol tissue homogenates. We performed inoculations, clinical examinations, sampling, neuropathologic diagnosis, and WB analysis of PrPSc as previously described (10).
Amplification of CWD Strains by PMCA Using Bank Vole Substrate
WBs of brain homogenates of the animals used in this study confirmed the presence or absence of PrPSc in the materials. This analysis highlighted the presence of various types of PrPres in relation to the known differences among species and within moose samples, using Sha31 antibodies, targeting the protein core, and 12B2 antibodies, targeting the N-terminal part of PrP protein (Figure 1, panel A). As expected, we detected PrPres in the brain of all CWD-affected animals, with the exception of reindeer B, where we detected it only in the lymph node by ELISA. The WB analysis showed the typical molecular profile of PrPres of the different strains, with banding patterns distinct across species, and among the moose with different genotypes. We observed a noticeable lower migration of the PrPres bands and a substantial reduction of signal with the N-terminal antibody in moose A and B, as described in Pirisinu et al. (13), and in red deer, indicative of a truncation of the N terminus of PrPres in these animals (Figure 1, panel A). In contrast to results for moose A and B, PrPres in moose C and D were not N-terminal truncated (17). Those results confirmed the known PrPSc variability in CWD isolates from different cervid species in Norway and showed that the moose samples included in this study have 2 different PrPSc types.
We assessed the efficiency of the bank vole substrate in amplifying the Norway CWD prions by subjecting 2 dilutions (10−4 and 10−8, vol/vol) of infected brains to PMCA amplifications. We also included brain homogenates from healthy animals as negative controls. After 4 rounds of amplification, WB analysis of the products revealed PrPres from all infected brain dilutions, indicating that the PMCA protocol used was successfully amplifying prions in reindeer, moose, and red deer, regardless of the CWD prion strains (Figure 1, panel B). We were unable to amplify PrPSc from the negative controls. Of interest, we also amplified PrPSc from the brain of reindeer B, an animal that had tested positive only in lymphoid tissue with traditional diagnostic tests.
PrPSc Detected in Lymph Nodes and Peripheral Nerves of CWD-Affected Cervids
Our primary objective was to investigate the lymphatic dissemination of CWD prions in moose and red deer by PMCA. To assess the presence of potential tissue inhibitors in the amplification process, we spiked brain-derived CWD reindeer prions in tissue homogenates from healthy reindeer and then subjected them to PMCA. Results showed that spiked CWD prions in muscle and lymph node homogenates induced a similar amplification efficiency as those in brain homogenate (Appendix Figure 1), suggesting the lack of tissue-specific inhibitors in the samples.
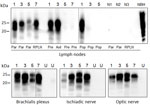
WB analysis of available lymph nodes collected from the head (parotid, retropharyngeal) or body (prescapular, axillary, and popliteal) before PMCA showed detection of PrPres only in reindeer, and in none of the other samples (Appendix Figure 2, panel A). Strikingly, we also detected seeding activity in the red deer and moose after amplification. In those cases, we were able to detect PrPres more readily in lymph nodes from the head than those located in the body (Table 2; Figure 2).
To examine the neural distribution of PrPSc in sites peripheral to the CNS, which was not detectable in moose and the red deer (Appendix Figure 2, panel B), we performed PMCA on the brachialis plexus, ischiadic nerve, and the optic nerve. The results showed prion amplification in all tested nerves (Figure 2).
Detection of PrPSc Seeding Activity in Muscle Tissues of CWD-Affected Cervids
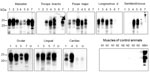
Because of the popularity of venison in the human food chain, it is important to assess the presence of prions in the musculature of CWD-affected cervids. We examined 8 different muscles (Table 2), all of which were negative for PrPres by direct WB (Appendix Figure 2, panel C). By PMCA, we amplified PrPSc from all muscle types tested, although with some individual variation (Table 2; Figure 3). Most muscles tested positive in reindeer and in 3 of 4 moose. We observed less positive PrPSc results in muscles from moose D and the red deer. Of the different muscles tested, masseter, ocular and lingual muscles from the head were always positive. In contrast, muscles located more peripherally from the head were negative in some animals. The semitendinosus muscle was the least frequently positive; only 2 of 6 samples tested positive. The efficiency of prion detection in skeletal muscles in moose, the red deer, and reindeer were similar in both laboratories, Ås and Milan, showing 92% agreement in the PMCA results (Appendix Table).
Bioassay of Peripheral Tissues from Moose in Bank Voles
To determine whether prions amplified in peripheral tissues by PMCA are also infectious, we intracerebrally inoculated Bv109I animals with selected tissues (Table 3). We chose moose and red deer tissues because of the high susceptibility of Bv109I voles to CWD strains in those species (10,18), which we confirmed by the efficient transmission of the moose C brain homogenate (Table 3), compared with the very low susceptibility observed for reindeer CWD (10). Of the 7 peripheral tissues tested, the ocular muscle and parotideal lymph node from moose A transmitted the disease in Bv109I voles, but with a lower attack rate and longer survival time compared with the brain of the same moose (Table 3). We observed no evidence of infectivity in the popliteal lymph node and longissimus dorsi muscle of moose A, the prescapular lymph nodes of moose C and the red deer, and the longissimus dorsi muscle of moose C.
Animals affected by CWD can remain asymptomatic for months, during which time prions can spread to various tissues and be shed into the environment, contributing to horizontal transmission (1). Depending on the prion strains, the PrPSc distribution pattern and tissue accumulation might differ in the host (24). Reports have identified 2 distinct patterns of prion spread in animal prion diseases (6,27–30). The first pattern involves the initial replication of prions in tissues from the lymphoreticular system, followed by spread to the peripheral nervous system before entering the brain. This pattern is observed in contagious strains, such as classical scrapie and North American CWD. The second pattern involves prion replication primarily in the CNS, as observed in less contagious or noncontagious prion diseases, such as BSE and Nor98/atypical scrapie.
There is limited research on the tissue distribution of the infectious agent in the newly emerging CWD strains in cervids in Norway. Through conventional diagnostic methods, investigators detected PrPSc in the lymph nodes of all reindeer with CWD but detected the prion in the CNS of only 50% of them. Conversely, researchers detected PrPSc in only the CNS in moose and the red deer (13,15). Using conventional WB in this study, we confirmed the presence of PrPres in brain, lymph nodes, and nerves in reindeer, but only moose and the red deer demonstrated evidence of PrPres in the brain (Figure 1, panel A; Appendix Figure 1). However, by using PMCA, we were able to demonstrate PrPSc seeding activity in all 3 species in various peripheral tissues, including the peripheral nerves, lymph nodes, and a variety of muscles. This finding illustrates the shortcomings of the conventional diagnostic methods in detecting minute quantities of prions, which impedes the accurate assessment of peripheral CWD distribution (31,32). Previous studies have demonstrated the presence of prions in multiple peripheral tissues and body fluids, including lymphoid tissues, peripheral nerves, muscles, blood, and excreta, in animals infected with North America CWD strains (20,22,33–38). The tissue distribution of PrPSc in reindeer, with a CWD strain similar to cases found in North America, was therefore not surprising. However, the findings of PrPSc in peripheral tissues in moose and red deer by PMCA were less expected, especially in muscles, given the sporadic occurrence and lack of evidence, to date, for contagiousness of these new CWD strains (11).
We confirmed the presence of prion infectivity in PMCA-positive lymphoid and muscle tissues by transmission in bank voles. Despite the limited number of samples examined, infectivity and seeding activity showed a good correlation: 2 of 4 positive transmissions from PMCA-positive samples and 0 of 3 from PMCA-negative samples. This correlation suggested PMCA to be a good proxy for CWD infectivity in bank voles, as observed in sheep scrapie (39). The negative transmission observed with 2 PMCA-positive tissues could be the result of the presence of very low levels of infectivity, below the detection limit of the vole bioassay. This finding is consistent with the partial transmission rate observed in the positive transmissions.
Identifying prions outside of CNS in cases of CWD in moose and the red deer mirrors the pattern observed in Nor98/atypical scrapie in sheep and atypical BSE forms in cattle, which represent additional sporadic animal prion diseases. Initially, researchers believed the accumulation of PrPSc to be confined to the CNS in these diseases (27). Nevertheless, subsequent studies using bioassay experiments demonstrated that infectivity could be detected in peripheral tissues that had been considered negative by traditional PrPSc detection techniques, including lymphoid tissues, nerves, and muscles (40,41).
Transmission studies have reported the presence of PrPSc in muscles in hamsters and sheep orally challenged with classical scrapie and, subsequently, in CWD-infected deer (27). Other studies reported detection of PrPSc in muscles of intracerebrally inoculated hamsters and mice, as well as in patients with Creutzfeldt-Jakob disease (42,43). In those instances, PrPSc may have reached the muscles via centrifugal spread, through peripheral nerves and the innervation of efferent and sensory nerve fibers to the tissues (27). Our investigation indicated that a similar phenomenon might occur in Norway strains, regardless of the cervid species involved. Of note, we observed a more efficient amplification of PrPSc in muscle and lymph node samples that were closer to the head and CNS compared with those further apart. Similarly, we detected infectivity in tissues from the head, the parotid lymph node, and the ocular muscle from moose A; however, the PMCA-positive popliteal lymph node and longissimus muscle from the same moose did not transmit in bank voles. Considering the incomplete transmission rate we observed in analyzing positive transmissions from peripheral tissues compared with the brain, we theorize that CWD prions accumulate at low titers in peripheral tissues and that prion titers might be higher in tissues closer to the CNS.
Unfortunately, in examining only field cases in our study, we concede that the lack of clinical status of the animals precludes establishing a correlation between the peripheral distribution of PrPSc and the disease stage. For instance, we were unable to determine whether the absence of seeding activity in some specific muscles and lymph nodes was a result of these animals being culled during early stages of the disease. We also could not rule out that the observed variation could be the result of very low prion titers in peripheral tissues or of individual strain characteristics.
In summary, the results of our study indicate that prions are widely distributed in peripheral and edible tissues of cervids in Norway, including muscles. This finding highlights the risk of human exposure to small amounts of prions through handling and consuming infected cervids. Nevertheless, we note that this study did not investigate the zoonotic potential of the Norway CWD prions. In North America, humans have historically consumed meat from CWD-infected animals, which has been documented to harbor prions (35,44–47). Despite the potential exposure to prions, no epidemiologic evidence indicates a correlation between the occurrence of CWD cases in animals and the prevalence of human prion diseases (48). A recent bioassay study reported no transmissions from 3 Nordic isolates into transgenic mice expressing human PrP (49). Therefore, our findings should be interpreted with caution in terms of human health implications, and further research is required to determine the zoonotic potential of these CWD strains.
The presence of prions in peripheral tissues indicates that CWD may have a systemic nature in all Norwegian cervid species, challenging the view that prions are exclusively localized in the CNS in sporadic CWD of moose and red deer. Our findings expand the notion of just how widely distributed prions can be in cervids affected with CWD and call into question the capability of emerging CWD strains in terms of infectivity to other species, including humans.
Dr. Vuong is a research scientist at Norwegian Veterinary Institute in Ås, Norway. Her research interests are the pathogenesis of chronic wasting disease and the development of biochemical techniques to detect prions.
Acknowledgment
This study was funded by the internal CWD project 12081 at the Norwegian Veterinary institute, Research Council of Norway project no. 334585, and Italian Ministry of Health (to F.M.).
References
- Otero A, Velásquez CD, Aiken J, McKenzie D. Chronic wasting disease: a cervid prion infection looming to spillover. Vet Res. 2021;52:115. DOIPubMedGoogle Scholar
- Sejvar JJ, Schonberger LB, Belay ED. Transmissible spongiform encephalopathies. J Am Vet Med Assoc. 2008;233:1705–12. DOIPubMedGoogle Scholar
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. DOIPubMedGoogle Scholar
- US Geological Survey National Wildlife Health Center. Distribution of chronic wasting disease in North America [cited 2024 Jun 20] https://www.usgs.gov/centers/nwhc/science/expanding-distribution-chronic-wasting-disease
- Tranulis MA, Gavier-Widén D, Våge J, Nöremark M, Korpenfelt S-L, Hautaniemi M, et al. Chronic wasting disease in Europe: new strains on the horizon. Acta Vet Scand. 2021;63:48. DOIPubMedGoogle Scholar
- Sun JL, Kim S, Crowell J, Webster BK, Raisley EK, Lowe DC, et al. Novel prion strain as cause of chronic wasting disease in a moose, Finland. Emerg Infect Dis. 2023;29:323–32. DOIPubMedGoogle Scholar
- Sola D, Tran L, Våge J, Madslien K, Vuong TT, Korpenfelt SL, et al. Heterogeneity of pathological prion protein accumulation in the brain of moose (Alces alces) from Norway, Sweden and Finland with chronic wasting disease. Vet Res. 2023;54:74. DOIPubMedGoogle Scholar
- Bian J, Kim S, Kane SJ, Crowell J, Sun JL, Christiansen J, et al. Adaptive selection of a prion strain conformer corresponding to established North American CWD during propagation of novel emergent Norwegian strains in mice expressing elk or deer prion protein. PLoS Pathog. 2021;17:
e1009748 . DOIPubMedGoogle Scholar - Nonno R, Di Bari MA, Pirisinu L, D’Agostino C, Vanni I, Chiappini B, et al. Studies in bank voles reveal strain differences between chronic wasting disease prions from Norway and North America. Proc Natl Acad Sci U S A. 2020;117:31417–26. DOIPubMedGoogle Scholar
- Hopp P, Rolandsen CM, Korpenfelt SL, Våge J, Sörén K, Solberg EJ, et al. Sporadic cases of chronic wasting disease in old moose - an epidemiological study. J Gen Virol. 2024;105:105. DOIPubMedGoogle Scholar
- Di Bari MA, Nonno R, Castilla J, D’Agostino C, Pirisinu L, Riccardi G, et al. Chronic wasting disease in bank voles: characterisation of the shortest incubation time model for prion diseases. PLoS Pathog. 2013;9:
e1003219 . DOIPubMedGoogle Scholar - Pirisinu L, Tran L, Chiappini B, Vanni I, Di Bari MA, Vaccari G, et al. Novel type of chronic wasting disease detected in moose (Alces alces), Norway. Emerg Infect Dis. 2018;24:2210–8. DOIPubMedGoogle Scholar
- Koutsoumanis K, Allende A, Alvarez-Ordoňez A, Bolton D, Bover-Cid S, Chemaly M, et al.; EFSA Panel on Biological Hazards (BIOHAZ). Update on chronic wasting disease (CWD) III. EFSA J. 2019;17:
e05863 .PubMedGoogle Scholar - Vikøren T, Våge J, Madslien KI, Røed KH, Rolandsen CM, Tran L, et al. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J Wildl Dis. 2019;55:970–2. DOIPubMedGoogle Scholar
- Pritzkow S, Gorski D, Ramirez F, Telling GC, Benestad SL, Soto C. North American and Norwegian chronic wasting disease prions exhibit different potential for interspecies transmission and zoonotic risk. J Infect Dis. 2022;225:542–51. DOIPubMedGoogle Scholar
- Harpaz E, Vuong TT, Tran L, Tranulis MA, Benestad SL, Ersdal C. Inter- and intra-species conversion efficacies of Norwegian prion isolates estimated by serial protein misfolding cyclic amplification. Vet Res. 2023;54:84. DOIPubMedGoogle Scholar
- Marín-Moreno A, Benestad SL, Barrio T, Pirisinu L, Espinosa JC, Tran L, et al. Classical BSE dismissed as the cause of CWD in Norwegian red deer despite strain similarities between both prion agents. Vet Res. 2024;55:62. DOIPubMedGoogle Scholar
- Race BL, Meade-White KD, Ward A, Jewell J, Miller MW, Williams ES, et al. Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerg Infect Dis. 2007;13:824–30. DOIPubMedGoogle Scholar
- Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One. 2009;4:
e5916 . DOIPubMedGoogle Scholar - John TR, Schätzl HM, Gilch S. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion. 2013;7:253–8. DOIPubMedGoogle Scholar
- Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, et al. Rapid antemortem detection of CWD prions in deer saliva. PLoS One. 2013;8:
e74377 . DOIPubMedGoogle Scholar - Koutsoumanis K, Allende A, Alvarez-Ordoñez A, Bolton D, Bover-Cid S, Chemaly M, et al.; EFSA Panel on Biological Hazards (BIOHAZ). Monitoring of chronic wasting disease (CWD) (IV). EFSA J. 2023;21:
e07936 .PubMedGoogle Scholar - Lambert ZJ, Greenlee JJ, Cassmann ED, West Greenlee MH. Differential accumulation of misfolded prion strains in natural hosts of prion diseases. Viruses. 2021;13:2453. DOIPubMedGoogle Scholar
- Simmons SM, Bartz JC. Strain-specific targeting and destruction of cells by prions. Biology (Basel). 2024;13:57. DOIPubMedGoogle Scholar
- Güere ME, Våge J, Tharaldsen H, Benestad SL, Vikøren T, Madslien K, et al. Chronic wasting disease associated with prion protein gene (PRNP) variation in Norwegian wild reindeer (Rangifer tarandus). Prion. 2020;14:1–10. DOIPubMedGoogle Scholar
- Beekes M, McBride PA. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274:588–605. DOIPubMedGoogle Scholar
- Fox KA, Jewell JE, Williams ES, Miller MW. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol. 2006;87:3451–61. DOIPubMedGoogle Scholar
- Hoover CE, Davenport KA, Henderson DM, Denkers ND, Mathiason CK, Soto C, et al. Pathways of prion spread during early chronic wasting disease in deer. J Virol. 2017;91:e00077–17. DOIPubMedGoogle Scholar
- Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol. 1999;80:2757–64. DOIPubMedGoogle Scholar
- Benavente R, Reed JH, Lockwood M, Morales R. PMCA screening of retropharyngeal lymph nodes in white-tailed deer and comparisons with ELISA and IHC. Sci Rep. 2023;13:20171. DOIPubMedGoogle Scholar
- Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol. 2011;85:6309–18. DOIPubMedGoogle Scholar
- Ferreira NC, Charco JM, Plagenz J, Orru CD, Denkers ND, Metrick MA II, et al. Detection of chronic wasting disease in mule and white-tailed deer by RT-QuIC analysis of outer ear. Sci Rep. 2021;11:7702. DOIPubMedGoogle Scholar
- Cooper SK, Hoover CE, Henderson DM, Haley NJ, Mathiason CK, Hoover EA. Detection of CWD in cervids by RT-QuIC assay of third eyelids. PLoS One. 2019;14:
e0221654 . DOIPubMedGoogle Scholar - Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, et al. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. DOIPubMedGoogle Scholar
- Henderson DM, Denkers ND, Hoover CE, McNulty EE, Cooper SK, Bracchi LA, et al. Progression of chronic wasting disease in white-tailed deer analyzed by serial biopsy RT-QuIC and immunohistochemistry. PLoS One. 2020;15:
e0228327 . DOIPubMedGoogle Scholar - Kramm C, Soto P, Nichols TA, Morales R. Chronic wasting disease (CWD) prion detection in blood from pre-symptomatic white-tailed deer harboring PRNP polymorphic variants. Sci Rep. 2020;10:19763. DOIPubMedGoogle Scholar
- Henderson DM, Tennant JM, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. Detection of chronic wasting disease prion seeding activity in deer and elk feces by real-time quaking-induced conversion. J Gen Virol. 2017;98:1953–62. DOIPubMedGoogle Scholar
- Chianini F, Cosseddu GM, Steele P, Hamilton S, Hawthorn J, Síso S, et al. Correlation between infectivity and disease associated prion protein in the nervous system and selected edible tissues of naturally affected scrapie sheep. PLoS One. 2015;10:
e0122785 . DOIPubMedGoogle Scholar - Andréoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, et al. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog. 2011;7:
e1001285 . DOIPubMedGoogle Scholar - Kumagai S, Daikai T, Onodera T. Bovine spongiform encephalopathy—a review from the perspective of food safety. Food Saf (Tokyo). 2019;7:21–47. DOIPubMedGoogle Scholar
- Peden AH, Ritchie DL, Head MW, Ironside JW. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:927–35. DOIPubMedGoogle Scholar
- Thomzig A, Cardone F, Krüger D, Pocchiari M, Brown P, Beekes M. Pathological prion protein in muscles of hamsters and mice infected with rodent-adapted BSE or vCJD. J Gen Virol. 2006;87:251–4. DOIPubMedGoogle Scholar
- Bosque PJ, Ryou C, Telling G, Peretz D, Legname G, DeArmond SJ, et al. Prions in skeletal muscle. Proc Natl Acad Sci U S A. 2002;99:3812–7. DOIPubMedGoogle Scholar
- Daus ML, Breyer J, Wagenfuehr K, Wemheuer WM, Thomzig A, Schulz-Schaeffer WJ, et al. Presence and seeding activity of pathological prion protein (PrP(TSE)) in skeletal muscles of white-tailed deer infected with chronic wasting disease. PLoS One. 2011;6:
e18345 . DOIPubMedGoogle Scholar - Jewell JE, Brown J, Kreeger T, Williams ES. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J Gen Virol. 2006;87:3443–50. DOIPubMedGoogle Scholar
- Li M, Schwabenlander MD, Rowden GR, Schefers JM, Jennelle CS, Carstensen M, et al. RT-QuIC detection of CWD prion seeding activity in white-tailed deer muscle tissues. Sci Rep. 2021;11:16759. DOIPubMedGoogle Scholar
- Tranulis MA, Tryland M. The zoonotic potential of chronic wasting disease—a review. Foods. 2023;12:824. DOIPubMedGoogle Scholar
- Wadsworth JDF, Joiner S, Linehan JM, Jack K, Al-Doujaily H, Costa H, et al. Humanized transgenic mice are resistant to chronic wasting disease prions from Norwegian reindeer and moose. J Infect Dis. 2022;226:933–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: January 10, 2025
1These first authors contributed equally to this article.
2These authors are co–senior authors.
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Tram Thu Vuong, Norwegian Veterinary Institute, Arboretveien 57, 1433 Ås, Norway
Top