Volume 31, Number 4—April 2025
Research
Detection and Decontamination of Chronic Wasting Disease Prions during Venison Processing
Figure 5
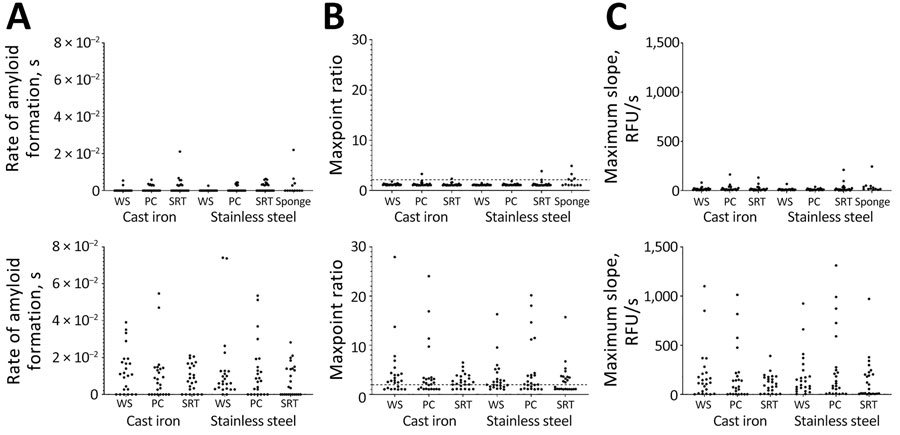
Figure 5. Results of real-time quaking-induced conversion conducted on meat grinder swab samples in study of detection and decontamination of chronic wasting disease prions during venison processing. A) Rate of amyloid formation; B) maxpoint ratio (ratio of the maximum value to the initial reading); C) maximum slope. Results are shown for negative controls (before meat grinder was used; top row) and after homogenate (after chronic wasting disease–positive muscle was passed through; bottom row). Each dot is an average of a single biologic replicate (consisting of 8 technical replicates). Each set of paired samples (e.g., cast iron worm spindles) resulted in a statistically significant difference post homogenate compared with the negative samples (p<0.05; data not shown). Dashed lines indicate the cutoff for significance using the maxpoint ratio (28). PC, plate cutter; SRT, screw ring threads; WS, worm spindle.
References
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. DOIPubMedGoogle Scholar
- Miller MW, Williams ES. Chronic wasting disease of cervids. In: Harris DA, editor. Mad cow disease and related spongiform encephalopathies. Berlin: Springer; 2004. p. 193–214.
- Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. DOIPubMedGoogle Scholar
- US Geological Survey. Distribution of chronic wasting disease in North America [cited 2024 May 22]. https://www.usgs.gov/media/images/distribution-chronic-wasting-disease-north-america-0
- National Deer Association. NDA’s deer report 2023 [cited 2024 Aug 7]. https://deerassociation.com/wp-content/uploads/2023/02/NDA-DR2023-FINAL.pdf
- Manitoba Wildlife Federation. The challenge of CWD: insidious, dire and urgent, late-breaking research reinforces the need for action [cited 2024 May 22]. https://mwf.mb.ca/archives/709
- Li M, Schwabenlander MD, Rowden GR, Schefers JM, Jennelle CS, Carstensen M, et al. RT-QuIC detection of CWD prion seeding activity in white-tailed deer muscle tissues. Sci Rep. 2021;11:16759. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. About chronic wasting disease (CWD) [cited 2024 May 22]. https://www.cdc.gov/chronic-wasting/about/index.html
- Tranulis MA, Tryland M. The zoonotic potential of chronic wasting disease—a review. Foods. 2023;12:824. DOIPubMedGoogle Scholar
- Otero A, Duque Velasquez C, McKenzie D, Aiken J. Emergence of CWD strains. Cell Tissue Res. 2023;392:135–48. DOIPubMedGoogle Scholar
- Cassmann ED, Frese AJ, Moore SJ, Greenlee JJ. Transmission of raccoon-passaged chronic wasting disease agent to white-tailed deer. Viruses. 2022;14:1578. DOIPubMedGoogle Scholar
- Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol. 2015;96:210–9. DOIPubMedGoogle Scholar
- Tennant JM, Li M, Henderson DM, Tyer ML, Denkers ND, Haley NJ, et al. Shedding and stability of CWD prion seeding activity in cervid feces. PLoS One. 2020;15:
e0227094 . DOIPubMedGoogle Scholar - Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–6. DOIPubMedGoogle Scholar
- Escobar LE, Pritzkow S, Winter SN, Grear DA, Kirchgessner MS, Dominguez-Villegas E, et al. The ecology of chronic wasting disease in wildlife. Biol Rev Camb Philos Soc. 2020;95:393–408. DOIPubMedGoogle Scholar
- Orrú CD, Groveman BR, Hughson AG, Barrio T, Isiofia K, Race B, et al. Sensitive detection of pathological seeds of α-synuclein, tau and prion protein on solid surfaces. PLoS Pathog. 2024;20:
e1012175 . DOIPubMedGoogle Scholar - Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol. 2006;87:3737–40. DOIPubMedGoogle Scholar
- Yuan Q, Rowden G, Wolf TM, Schwabenlander MD, Larsen PA, Bartelt-Hunt SL, et al. Sensitive detection of chronic wasting disease prions recovered from environmentally relevant surfaces. Environ Int. 2022;166:
107347 . DOIPubMedGoogle Scholar - Soto P, Bravo-Risi F, Benavente R, Lichtenberg S, Lockwood M, Reed JH, et al. Identification of chronic wasting disease prions in decaying tongue tissues from exhumed white-tailed deer. MSphere. 2023;8:
e0027223 . DOIPubMedGoogle Scholar - Chesney AR, Booth CJ, Lietz CB, Li L, Pedersen JA. Peroxymonosulfate rapidly inactivates the disease-associated prion protein. Environ Sci Technol. 2016;50:7095–105. DOIPubMedGoogle Scholar
- Williams K, Hughson AG, Chesebro B, Race B. Inactivation of chronic wasting disease prions using sodium hypochlorite. PLoS One. 2019;14:
e0223659 . DOIPubMedGoogle Scholar - Hughson AG, Race B, Kraus A, Sangaré LR, Robins L, Groveman BR, et al. Inactivation of prions and amyloid seeds with hypochlorous acid. PLoS Pathog. 2016;12:
e1005914 . DOIPubMedGoogle Scholar - Gavin C, Henderson D, Benestad SL, Simmons M, Adkin A. Estimating the amount of Chronic Wasting Disease infectivity passing through abattoirs and field slaughter. Prev Vet Med. 2019;166:28–38. DOIPubMedGoogle Scholar
- Nastasijevic I, Boskovic M, Glisic M. Abattoir hygiene. In: Knowles ME, Anelich LE, Boobis AR, Popping B, editors. Present knowledge in food safety: a risk-based approach through the food chain. San Diego: Academic Press; 2023. p. 412–38.
- Atarashi R, Sano K, Satoh K, Nishida N. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion. 2011;5:150–3. DOIPubMedGoogle Scholar
- Haley NJ, Van de Motter A, Carver S, Henderson D, Davenport K, Seelig DM, et al. Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PLoS One. 2013;8:
e81488 . DOIPubMedGoogle Scholar - Gallups NJ, Harms AS. ‘Seeding’ the idea of early diagnostics in synucleinopathies. Brain. 2022;145:418–9. DOIPubMedGoogle Scholar
- Rowden GR, Picasso-Risso C, Li M, Schwabenlander MD, Wolf TM, Larsen PA. Standardization of data analysis for RT-QuIC-based detection of chronic wasting disease. Pathogens. 2023;12:309. DOIPubMedGoogle Scholar
- Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, Race B, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6:
e1001217 . DOIPubMedGoogle Scholar - Cooke CM, Rodger J, Smith A, Fernie K, Shaw G, Somerville RA. Fate of prions in soil: detergent extraction of PrP from soils. Environ Sci Technol. 2007;41:811–7. DOIPubMedGoogle Scholar
- Pitardi D, Meloni D, Maurella C, Di Vietro D, Nocilla L, Piscopo A, et al. Specified risk material removal practices: can we reduce the BSE hazard to human health? Food Control. 2013;30:668–74. DOIGoogle Scholar