Volume 31, Number 4—April 2025
Research
Detection and Decontamination of Chronic Wasting Disease Prions during Venison Processing
Abstract
Prion diseases, including chronic wasting disease (CWD), are caused by prions, which are misfolded aggregates of normal cellular prion protein. Prions possess many characteristics that distinguish them from conventional pathogens, in particular, an extraordinary recalcitrance to inactivation and a propensity to avidly bind to surfaces. In middle to late stages of CWD, prions begin accumulating in cervid muscle tissues. Those features collectively create scenarios in which occupational hazards arise for workers processing venison and pose risks to consumers through direct prion exposure through ingestion and cross-contamination of food products. In this study, we demonstrate that steel and plastic surfaces used in venison processing can be directly contaminated with CWD prions and that cross-contamination of CWD-negative venison can occur from equipment that had previously been used with CWD-positive venison. We also show that several decontaminant solutions (commercial bleach and potassium peroxymonosulfate) are efficacious for prion inactivation on those same surfaces.
Chronic wasting disease (CWD) is a fatal prion disease affecting cervids caused by a self-templating, misfolded, and infectious form of the prion protein (PrPSc) (1). Since its discovery in the United States in the 1960s (2,3), CWD has been detected in free-ranging and captive cervid populations in 34 US states and 5 provinces in Canada, as well as Nordic countries and South Korea (4). CWD continues to spread in white-tailed deer (Odocoileus virginanus), mule deer (Odocoileus hemionus), moose (Alces alces), and elk (Cervus canadensis) populations across the United States and Canada. In the United States alone, >6 million white-tailed deer are harvested annually, many of which are consumed and represent a major source of protein for communities across the country (5). A 2017 estimate suggests that as many as 15,000 CWD-positive white-tailed deer are consumed in the United States annually (6). This number, however, is likely underestimated given the limitations of existing CWD surveillance programs and venison food donation efforts within CWD-endemic regions.
CWD prions accumulate in tissues during disease pathogenesis and can be detected in the muscle tissue of white-tailed deer (7); thus, consuming meat from CWD-positive cervids might expose humans to CWD prions. Scientific and public health communities are concerned about the potential transmission of CWD to humans, particularly through ingestion. The Centers for Disease Control and Prevention acknowledges this risk and recommends reducing risk by testing cervids before consuming meat, processing each animal individually to avoid cross-contamination, and not consuming CWD-positive meat (8). No cases of CWD in humans have been confirmed (9); however, as new CWD strains are identified (10) and more organisms are exposed to PrPSc (11), concerns are growing that the species barrier might be crossed.
When PrPSc prions are introduced into the environment through natural shedding (12,13) or carcass decomposition (14), they can adsorb to surfaces where they can be detected long after deposition (15–17). Surface swabbing is an effective CWD detection method for both laboratory settings (18) and natural, environmentally exposed surfaces (19). Yuan et al. (18) highlighted the importance of surface structure for prion recovery, noting that porous surfaces, such as wood, were ineffective for swab-based detection, as opposed to nonporous surfaces, such as glass and stainless steel. Those attributes (e.g., environmental stability, swab detection, surface adsorption) also factor into surface decontamination, because chemical decontaminants must physically contact PrPSc aggregates for disintegration or other forms of inactivation (20–22).
Venison processing for human consumption, both in-home and commercial, is an area of potential PrPSc cross-contamination and direct human exposure. Surfaces, tools, and equipment used for venison processing can be contaminated with PrPSc from CWD-positive venison (23,24). Cleaning strategies used during venison processing can vary widely and might not be effective in removing or destroying PrPSc, particularly in unregulated facilities or scenarios (e.g., home butchery, seasonal pop-up processors). Thus, understanding the potential of PrPSc to more widely enter the food supply through surface contamination and the efficacy of chemical decontamination are clearly needed.
In this study, we examined prion contamination of commonly used meat-processing equipment, including knives, cutting boards, and household-style meat grinders, and the efficacy of decontaminants commonly used in home or commercial processing. In addition, we investigated the cross-contamination of CWD-negative meat after contact with CWD-contaminated processing equipment.
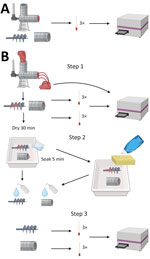
This work consisted of 5 distinct phases. The 5 phases were pilot study, study controls, knife and cutting board, meat cross-contamination, and meat grinder (Table 1, Figure 1; Appendix).
Pilot Study
We conducted a pilot study to determine whether we could recover, detect, and decontaminate prions on common meat-processing surfaces with known CWD-positive samples with different prion loads from white-tailed deer: cerebellum (high prion load) and muscle (low prion load). We conducted control experiments to determine whether the decontaminants could induce or suppress seeding activity during real-time quaking-induced conversion (RT-QuIC) (Appendix).
Study Controls
We performed experiments to test whether 5 chosen decontaminants would interfere with the RT-QuIC assay and whether those decontaminants would inhibit prion seeding activity on both stainless steel and cast iron surfaces. Chosen decontaminants were dish soap (Dawn brand; Procter and Gamble, https://dawn-dish.com), Briotech (0.02% hypochlorous acid solution; https://briotechusa.shop), Virkon-S (2% potassium peroxymonosulfate solution; Lanxess AG, https://lanxess.com), and 10% vol/vol (7,500 ppm) and 40% vol/vol (30,000 ppm) commercial bleach solution (7.5% sodium hypochlorite; The Clorox Company, https://www.clorox.com). In addition, we conducted experiments to assess the recovery and detection of CWD prions from stainless steel and cast iron surfaces.
Knife and Cutting Board
We sought to determine whether prion seeding activity could be detected before and after decontamination on stainless steel knives and polyethylene cutting boards, 2 standard pieces of equipment used in home and commercial meat processing. We designated a knife and cutting board for each of the 5 chosen decontaminants. We collected negative controls by swabbing the knife and cutting board before contact with any muscle samples.
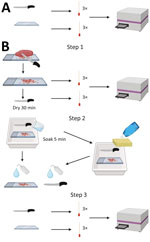
To test the efficacy of dish soap for prion decontamination, we made 2 cuts through CWD-negative muscle on the cutting board. We immediately swabbed the knife and cutting board after the first cut and left them to dry at room temperature for 30 minutes after the second cut. We filled a tray with dish soap and water according to manufacturer recommendations, scrubbed the knife and cutting board with a sponge (3M, https://www.scotch-brite.com), and then rinsed with low-pressure municipal cold water. We then swabbed the surfaces again. We repeated the experiment using CWD-positive muscle and new materials. We stored all swabs at −80°C until use.
To test the efficacy of Briotech, 2% Virkon-S, 10% vol/vol (7,500 ppm) bleach solution and 40% vol/vol (30,000 ppm) bleach solution, we followed the same procedure as described but did not perform the sponge step. Instead, after drying the polyethylene cutting board and knife for 30 minutes, we soaked the items in the decontaminant solution for 5 minutes. Then, we rinsed the knife and cutting board with water and swabbed them again (Figure 1).
Meat Cross-Contamination
We passed CWD-positive muscle samples through a meat grinder several times to produce a homogenized pool of CWD-positive muscle, which we then subsampled. We used that pool of CWD-positive material for the meat grinder experiments. We disassembled the grinder, removed the gross material, and swabbed the parts. We then reassembled the grinder, passed CWD-negative muscle through the grinder, and subsampled.
We performed an endpoint titration on the homogenized CWD-positive muscle pool to determine prion load relative to our positive lymph node control. We tested the muscle pool at dilutions from 10–1 to 10–9. We observed statistically significant seeding activity at 10–1 and 10–2 dilution and nonsignificant seeding activity at 10–3 dilution. We observed no seeding activity in the pool at dilutions 10–4 through 10–9. Comparatively, our positive lymph node control has historically shown seeding activity through a dilution of 10–6.
Meat Grinder
On the basis of preliminary results demonstrating the efficacy of the decontaminants used in the knife and polyethylene cutting board study, we chose the following 4 decontaminants to be used in experiments using meat grinders: dish soap, Virkon-S, 10% (7,500 ppm) bleach solution, and 40% (30,000 ppm) bleach solution. We used stainless steel (CHOLISM) and cast iron (CucinaPro, https://cucinapro.com) meat grinders with each decontaminant. We disassembled each grinder and swabbed the worm spindle, plate cutter holes, and screw ring threads before contact with CWD-positive muscle. The experiments were designed to mimic home or small-scale commercial meat processing.
Cleaning of Grinder after CWD-Positive Homogenate
After each grinder was assembled, we passed through CWD-positive homogenate and then allowed the grinder to air dry for 30 minutes. We then disassembled the grinders, removed gross material, and swabbed the grinder parts again. We then placed the parts in a tray with diluted dish soap, used a sponge to wash them, and rinsed them with water. We sampled the sponge and swabbed the grinder parts. For the remaining 3 decontaminants (Virkon-S, 10% [7,500 ppm] bleach, and 40% [30,000 ppm] bleach), we left the grinder parts to soak for 5 minutes (instead of washing with a sponge), rinsed them with low-pressure cold water, and swabbed in the same locations (Figure 2). Swab extraction and processing followed the procedure of Yuan et al. (19) with some modifications (Appendix).
Analysis
Fluorescence readout from the RT-QuIC assay captures the kinetics of amyloid formation in vitro (25). Three metrics can be used to describe data generated by RT-QuIC: rate of amyloid formation (RAF) for nucleation, maximum slope (MS) for elongation (26,27), and maxpoint ratio (MPR) for equilibrium (28). We calculated RAF per well as the reciprocal of the time required for fluorescence to reach a threshold of twice the background fluorescence. If a well did not reach the threshold, we assigned a value of zero. We calculated background fluorescence as the average fluorescent reading per well at cycles 2 and 3 to control for any variability in relative fluorescence units (RFU) among wells and to compensate for the greater ThT fluorescence typically found in the first cycle because of viscosity effects. We calculated the slope as the difference between the RFU at the current time position plus 6 cycles (3.75 hours) divided by the time. MPR was calculated as the maximum RFU divided by the background RFU. Using all 3 metrics, genuine amyloid formation of PrPSc induced by CWD prions in RT-QuIC reactions should generate responses that are significantly different from reactions with no seeding activities.
A member of the research team (M.L.), blinded to sample identity and treatment, analyzed the data generated from the pilot study. Using a 1-tailed Wilcoxon Rank Sum test, with an ɑ-level of 0.05, we compared RAF, MPR, and MS against the negative plate controls. M.L. and unblinded team members (M.M., S.G.) reviewed the results from the pilot data and set the following swab inclusion criteria for the remainder of the study: all 3 metrics (RAF, MPR, MS) must be statistically significantly higher than the negative plate controls to be considered positive, and regardless of dilution level, if a sample meets the first criterion, the sample will be considered positive. We performed all statistical analysis for swab samples in R version 4.3.2 (The R Project for Statistical Computing, https://www.r-project.org).
For all muscle sample analyses, we used an uncorrected Fisher LSD test with a single pooled variance (α level of 0.05). We compared RAF, MPR, and MS against the negative plate controls. All 3 metrics (RAF, MPR, MS) must be statistically significantly higher than the negative plate controls to be considered positive. If a sample met those criteria, it was considered positive regardless of the dilution level. We performed all statistical analyses of muscle samples using GraphPad Prism version 10.0.2 (https://www.graphpad.com).
Pilot Study
Multiple swabs demonstrated significant seeding activity in the RT-QuIC assay: knife blade swabs and polyethylene cutting board swabs from cutting CWD-positive muscle and knife blade swabs and cutting board swabs from cutting CWD-positive brain. After soaking the knife and cutting board with 3 dilutions of household bleach (10%, 40%, 100%), all posttreatment swabs (n = 30; 2 or 3 knife swabs and 3 cutting board swabs per dilution, per sample type) were negative by RT-QuIC.
Study Controls
All negative controls (both knife and polyethylene cutting board) were negative by RT-QuIC for each of the 5 decontaminants after treatment (Tables 2,3). For each of the 5 decontaminants (dish soap, Briotech, Virkon-S, 10% [7,500 ppm] bleach, 40% [30,000 ppm] bleach), all samples had no significant seeding activity when CWD-negative muscle tissue was used during knife and cutting board experiments (Tables 2,3).
Knife and Cutting Board
Dish Soap
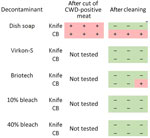
We detected significant seeding activity on the stainless steel knife and polyethylene cutting board after cutting CWD-positive muscle (Figure 3). We also detected significant seeding activity on the cutting board after cleaning with dish soap but not on the knife or sponge after cleaning with dish soap (Figure 3; sponge data not shown).
Briotech, 2% Virkon-S, 10% Bleach, 40% Bleach
After cleaning the CWD-contaminated polyethylene cutting board with Briotech, we detected significant seeding activity in 1 sample (Figure 3). We detected no significant seeding activity on CWD-contaminated knives after cleaning with any of the decontaminants (Figure 3) or on CWD-contaminated cutting boards after decontamination with Virkon-S, 10% (7,500 ppm) bleach, or 40% (30,000 ppm) bleach (Figure 3).
Meat Cross-Contamination
We detected an 88% significant seeding rate in the CWD-positive muscle homogenate pool (7/8 biological replicates) (Figure 4). When we passed CWD-positive muscle homogenate through a grinder, we detected significant seeding activity on the plate cutter (2/3 biological replicates, 66.7%) and screw ring thread (1/3 biological replicates, 33.3%). We detected no seeding activity on the worm spindle of the grinder. When we passed CWD-negative muscle through the grinder after the CWD-positive muscle homogenate, we found a 63% seeding activity rate (5/8 biological replicates) (Figure 4).
Meat Grinder
All negative controls of the stainless steel and cast iron grinder parts were negative by RT-QuIC (Figure 5). Significant seeding activity was demonstrated from swabs from all stainless steel and cast iron grinder parts after passage of CWD-positive muscle homogenate (Figures 5, 6). Significant seeding activity was demonstrated from multiple swabs after decontamination of the stainless steel and cast iron grinder parts with dish soap and Virkon-S, whereas no significant seeding activity was demonstrated from swabs after decontamination with 10% (7,500 ppm) and 40% (30,000 ppm) bleach (Figures 6, 7).
In this study, we examined the contamination of commonly used meat-processing equipment with CWD prions from CWD-positive muscle and the efficacy of decontaminants commonly used in homes or previously shown to have variable levels of efficacy for prion decontamination (20–22). In addition, we investigated the cross-contamination of meat in CWD prion–contaminated meat-processing equipment. Using a conservative approach in determining whether samples were positive (by requiring positivity using all 3 RT-QuIC metrics), we found that CWD prion seeding activity can be detected on common meat-processing surfaces after coming into contact with CWD-positive white-tailed deer muscle; CWD prion seeding activity can be transferred to CWD-negative meat after passing through a contaminated meat grinder; Virkon S, 10% bleach, and 40% bleach were effective for CWD prion decontamination on surfaces, as shown by removal of seeding activity; and surface composition might play a role in CWD prion detection and decontamination on meat-processing equipment.
On the basis of our results, CWD prion seeding activity is clearly detectable on common meat-processing equipment, such as knives, polyethylene cutting boards, and multiple parts of meat grinders. We would note that the presence of seeding activity does not directly imply infectivity, and the relationship between seeding activity and infectivity can be complicated. However, given that seeding activity is correlated with infectivity (29), and we are using materials that have verification of PrPSc presence by other means, the presence or absence of seeding activity in our study can be reasonably be concluded to correlate with the presence or absence of infectivity. Further investigation is warranted into the contamination regimes and decontaminants described using animal bioassays.
Of note, seeding activity was demonstrated not only in tissues containing high levels of prions (i.e., brain from a CWD-positive animal) but also in muscles with progressively lower levels of prions from a clinical deer and 2 hunter-harvested deer. We also detected seeding activity in the CWD-negative muscle homogenate after it passed through the contaminated meat grinder. Those findings exemplify real-life scenarios with implications for the food supply in which surfaces and tools are reused between deer and meat is mixed from multiple deer, potentially with nonclinical, untested deer.
Except for dish soap, all of the decontamination agents used in this study have been shown to inactivate prions (20–22); the active ingredient in Briotech is hypochlorous acid (22) and the active ingredient in Virkon-S is peroxymonosulfate (20). We found that 10% bleach and 40% bleach were highly effective at decontaminating the meat-processing surfaces tested in this study; Virkon-S was slightly less effective. Briotech was less effective and inconsistent in decontamination. Although the overall findings are promising, further investigation is warranted, especially given the lower relative prion load in our CWD-positive muscle pool used in the meat grinder studies. Decontamination efficacy on grinder parts could be further investigated by using tissues with prion loads higher than that typical in muscle. Reduced concentrations of bleach and contact times could still result in effective decontamination, but we did not test the lowest effective duration or concentration of decontaminants, nor did we test how repeated disinfection with the solutions affects surface integrity and ongoing disinfection efficacy. This factor is noteworthy because the chemicals can compromise the integrity of surfaces such as stainless steel, leading to unknown consequences of prion adsorption and decontamination. In addition, although we observed a few negative control swabs of grinder parts that were positive on initial test but negative upon retest, we hypothesize that those interferences arose from grease or metallic debris from the machining of parts. From a cleaning protocol perspective, the scrubbing of tools, parts, and surfaces is a key step, because tissue debris tends to remain on some surfaces after a decontamination soak. As demonstrated by others (21), decontaminants are ineffective at penetrating tissue, so removal of the tissue remnants will lead to more effective decontamination and reduce cross-contamination. This factor is one plausible explanation for the reduced efficacy (i.e., retained seeding activity) of Virkon-S in one of the treatments. Containing and disposing of the predecontamination cleaning wash is also a consideration for environmental prion contamination.
Of note, we saw differences in the detection of prion seeding activity between the knife and cutting board; after decontamination with dish soap, we were able to detect prion seeding on the surface of the cutting board but not on the surface of the knife. Curiously, we did not detect prion seeding activity in any of the sponge samples. We cannot discount the possibility that prions were removed from the knife surface by dish soap and simply remained in the decontaminant solution, speaking back to our point regarding containment and disposal of the cleaning solution itself. The principal components of dish soap are surfactants (30), which can remove adsorbed prions from surfaces, resulting in a lack of detection on the steel surface while also diluting prions to the point that they are undetectable by RT-QuIC. Those factors must all be considered when planning and implementing cleaning and decontamination of environmental prions.
In conclusion, our results show that processing of CWD-positive cervids for consumption has the potential to contaminate meat-processing equipment and cross-contaminate downstream meat products. Further, our data and previous studies (20,21) indicate that Virkon-S and bleach solutions with appropriate contact times (as little as 5 minutes) can effectively decontaminate nonporous surfaces of CWD prions. After the bovine spongiform encephalopathy outbreak in Europe in the early 1990s, processing practices became mandatory to remove and avoid specified risk materials (e.g., spinal cord, brain) and incorporate downstream molecular testing procedures to identify contamination in meat products (31). Our findings indicate similar practices may be necessary to reduce CWD exposure in humans through meat processing. However, given the unique features of CWD prions, contamination of equipment and surfaces is more challenging to control. This concern further highlights the importance of testing cervids before processing, in addition to the need for compliance with effective meat processing, environmental screening, waste management, and cleaning and decontamination protocols.
This article was published as a preprint at https://www.biorxiv.org/content/10.1101/2024.07.23.604851v1.
Dr. Milstein is a wildlife veterinary researcher at the University of Minnesota in St. Paul. Her primary research interests involve studying zoonotic disease transmission from the hunting, butchery, and consumption of wildlife.
Acknowledgments
We thank Davis Seelig and Russ Mason for their helpful discussions in planning this project. Sam Thomas kindly assisted with preliminary RT-QuIC experiments. We also thank Nicole Neeser and the Minnesota Department of Agriculture for guidance regarding meat processing and regulations. We are grateful to the Minnesota Department of Natural Resources, the University of Minnesota Veterinary Diagnostic Lab, and deer hunters for providing samples. We thank Suzanne Stone for laboratory management, Adam Reinschmidt for assisting with the pilot study, and Corina Valencia for sample management. Joel Pedersen contributed substantially to the acquisition of funding from the Michigan Department of Natural Resources.
This project was funded by the Minnesota legislature through the Rapid Agricultural Response Fund, the Michigan Department of Natural Resources, the Minnesota Agricultural Research, Education, Extension, and Technology Transfer (AGREETT) program, and the Minnesota Environment and Natural Resources Trust Fund, as recommended by the Legislative-Citizen Commission on Minnesota Resources (LCCMR).
P.A.L., S.-H.O., and M.D.S. are cofounders and stock owners of Priogen Corp, a diagnostic company specializing in the ultra-sensitive detection of pathogenic proteins associated with prion and protein-misfolding diseases. The University of Minnesota licensed patent applications to Priogen Corp. These interests have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies.
References
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. DOIPubMedGoogle Scholar
- Miller MW, Williams ES. Chronic wasting disease of cervids. In: Harris DA, editor. Mad cow disease and related spongiform encephalopathies. Berlin: Springer; 2004. p. 193–214.
- Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. DOIPubMedGoogle Scholar
- US Geological Survey. Distribution of chronic wasting disease in North America [cited 2024 May 22]. https://www.usgs.gov/media/images/distribution-chronic-wasting-disease-north-america-0
- National Deer Association. NDA’s deer report 2023 [cited 2024 Aug 7]. https://deerassociation.com/wp-content/uploads/2023/02/NDA-DR2023-FINAL.pdf
- Manitoba Wildlife Federation. The challenge of CWD: insidious, dire and urgent, late-breaking research reinforces the need for action [cited 2024 May 22]. https://mwf.mb.ca/archives/709
- Li M, Schwabenlander MD, Rowden GR, Schefers JM, Jennelle CS, Carstensen M, et al. RT-QuIC detection of CWD prion seeding activity in white-tailed deer muscle tissues. Sci Rep. 2021;11:16759. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. About chronic wasting disease (CWD) [cited 2024 May 22]. https://www.cdc.gov/chronic-wasting/about/index.html
- Tranulis MA, Tryland M. The zoonotic potential of chronic wasting disease—a review. Foods. 2023;12:824. DOIPubMedGoogle Scholar
- Otero A, Duque Velasquez C, McKenzie D, Aiken J. Emergence of CWD strains. Cell Tissue Res. 2023;392:135–48. DOIPubMedGoogle Scholar
- Cassmann ED, Frese AJ, Moore SJ, Greenlee JJ. Transmission of raccoon-passaged chronic wasting disease agent to white-tailed deer. Viruses. 2022;14:1578. DOIPubMedGoogle Scholar
- Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol. 2015;96:210–9. DOIPubMedGoogle Scholar
- Tennant JM, Li M, Henderson DM, Tyer ML, Denkers ND, Haley NJ, et al. Shedding and stability of CWD prion seeding activity in cervid feces. PLoS One. 2020;15:
e0227094 . DOIPubMedGoogle Scholar - Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–6. DOIPubMedGoogle Scholar
- Escobar LE, Pritzkow S, Winter SN, Grear DA, Kirchgessner MS, Dominguez-Villegas E, et al. The ecology of chronic wasting disease in wildlife. Biol Rev Camb Philos Soc. 2020;95:393–408. DOIPubMedGoogle Scholar
- Orrú CD, Groveman BR, Hughson AG, Barrio T, Isiofia K, Race B, et al. Sensitive detection of pathological seeds of α-synuclein, tau and prion protein on solid surfaces. PLoS Pathog. 2024;20:
e1012175 . DOIPubMedGoogle Scholar - Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol. 2006;87:3737–40. DOIPubMedGoogle Scholar
- Yuan Q, Rowden G, Wolf TM, Schwabenlander MD, Larsen PA, Bartelt-Hunt SL, et al. Sensitive detection of chronic wasting disease prions recovered from environmentally relevant surfaces. Environ Int. 2022;166:
107347 . DOIPubMedGoogle Scholar - Soto P, Bravo-Risi F, Benavente R, Lichtenberg S, Lockwood M, Reed JH, et al. Identification of chronic wasting disease prions in decaying tongue tissues from exhumed white-tailed deer. MSphere. 2023;8:
e0027223 . DOIPubMedGoogle Scholar - Chesney AR, Booth CJ, Lietz CB, Li L, Pedersen JA. Peroxymonosulfate rapidly inactivates the disease-associated prion protein. Environ Sci Technol. 2016;50:7095–105. DOIPubMedGoogle Scholar
- Williams K, Hughson AG, Chesebro B, Race B. Inactivation of chronic wasting disease prions using sodium hypochlorite. PLoS One. 2019;14:
e0223659 . DOIPubMedGoogle Scholar - Hughson AG, Race B, Kraus A, Sangaré LR, Robins L, Groveman BR, et al. Inactivation of prions and amyloid seeds with hypochlorous acid. PLoS Pathog. 2016;12:
e1005914 . DOIPubMedGoogle Scholar - Gavin C, Henderson D, Benestad SL, Simmons M, Adkin A. Estimating the amount of Chronic Wasting Disease infectivity passing through abattoirs and field slaughter. Prev Vet Med. 2019;166:28–38. DOIPubMedGoogle Scholar
- Nastasijevic I, Boskovic M, Glisic M. Abattoir hygiene. In: Knowles ME, Anelich LE, Boobis AR, Popping B, editors. Present knowledge in food safety: a risk-based approach through the food chain. San Diego: Academic Press; 2023. p. 412–38.
- Atarashi R, Sano K, Satoh K, Nishida N. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion. 2011;5:150–3. DOIPubMedGoogle Scholar
- Haley NJ, Van de Motter A, Carver S, Henderson D, Davenport K, Seelig DM, et al. Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PLoS One. 2013;8:
e81488 . DOIPubMedGoogle Scholar - Gallups NJ, Harms AS. ‘Seeding’ the idea of early diagnostics in synucleinopathies. Brain. 2022;145:418–9. DOIPubMedGoogle Scholar
- Rowden GR, Picasso-Risso C, Li M, Schwabenlander MD, Wolf TM, Larsen PA. Standardization of data analysis for RT-QuIC-based detection of chronic wasting disease. Pathogens. 2023;12:309. DOIPubMedGoogle Scholar
- Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, Race B, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6:
e1001217 . DOIPubMedGoogle Scholar - Cooke CM, Rodger J, Smith A, Fernie K, Shaw G, Somerville RA. Fate of prions in soil: detergent extraction of PrP from soils. Environ Sci Technol. 2007;41:811–7. DOIPubMedGoogle Scholar
- Pitardi D, Meloni D, Maurella C, Di Vietro D, Nocilla L, Piscopo A, et al. Specified risk material removal practices: can we reduce the BSE hazard to human health? Food Control. 2013;30:668–74. DOIGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 13, 2025
Table of Contents – Volume 31, Number 4—April 2025
| EID Search Options |
|---|
|
|
|
|
|
|




Please use the form below to submit correspondence to the authors or contact them at the following address:
Peter Larsen, University of Minnesota, Department of Veterinary and Biomedical Sciences 1971 Commonwealth Ave, 239B Veterinary Science Bldg, St. Paul, MN 55108, USA
Top