Volume 31, Number 6—June 2025
Research
Long-Term Clinical Outcomes of Adults Hospitalized for COVID-19 Pneumonia
Figure 2
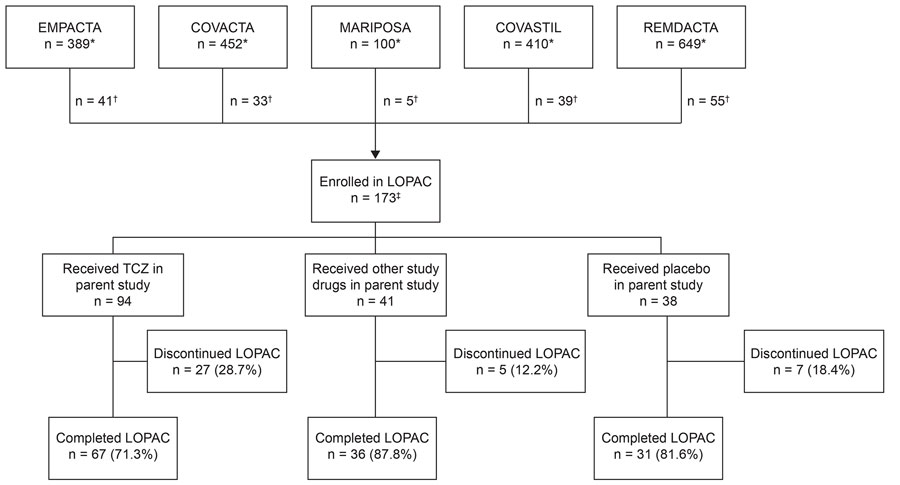
Figure 2. Disposition of LOPAC study participants in study of long-term clinical outcomes of adults hospitalized for COVID-19 pneumonia. The parent studies were clinical trials (registered with https://www.clinicaltrials.gov) as follows: EMPACTA (study no. NCT04372186), COVACTA (no. NCT04320615), MARIPOSA (no. NCT04363736), COVASTIL (no. NCT04386616), and REMDACTA (no. NCT04409262). Participants might have received remdesivir or another antiviral drug as part of the standard of care in the EMPACTA, COVACTA, and MARIPOSA studies. Participants who were randomly assigned to receive placebo, astegolimab, or efmarodocokin alfa might have also received TCZ or remdesivir or both as part of the standard of care in the COVASTIL study. Participants randomly assigned to receive TCZ or placebo in the REMDACTA study also received remdesivir. *Number of participants in each parent study; †number of participants from each parent study who participated in the LOPAC study; ‡1 participant initially enrolled in the LOPAC study did not receive treatment in the parent study and was not included in any analyses for the parent or LOPAC study. LOPAC, Long-Term Outcomes Post Acute COVID-19 study; TCZ, tocilizumab.