Volume 31, Number 6—June 2025
Research
Long-Term Clinical Outcomes of Adults Hospitalized for COVID-19 Pneumonia
Figure 5
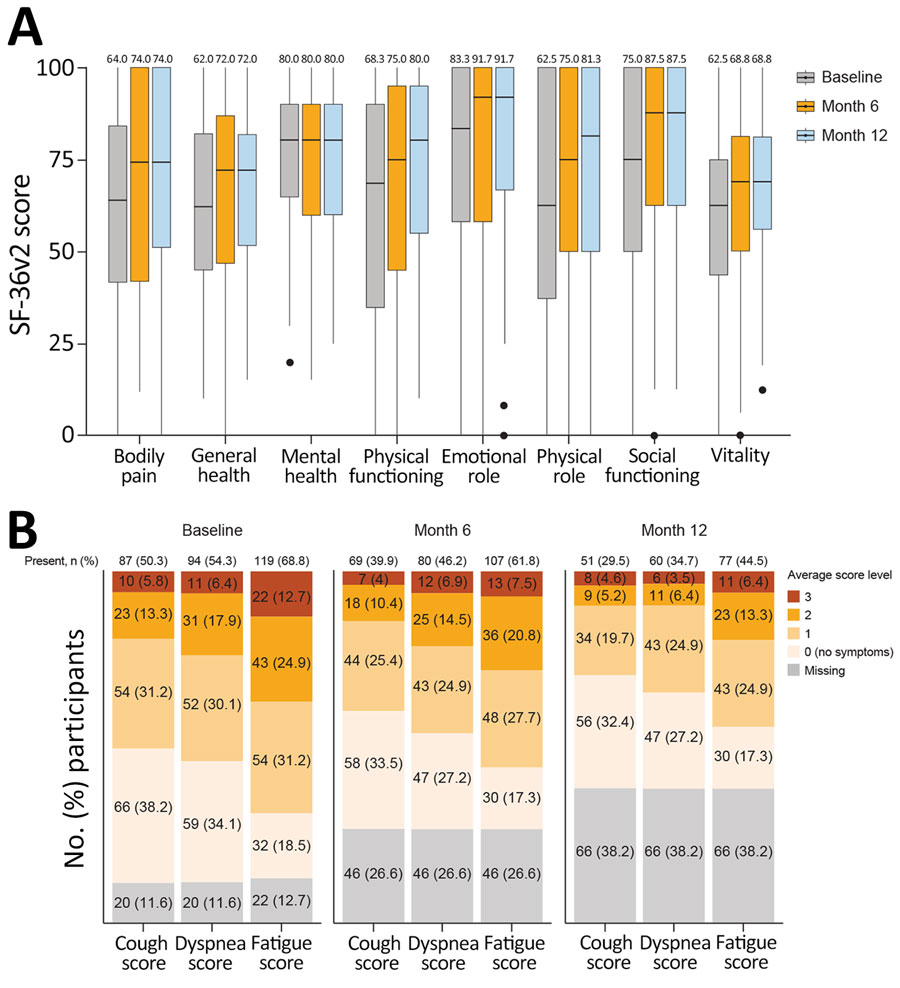
Figure 5. Longitudinal profiles of patient-reported outcomes from the Long-Term Outcomes Post Acute COVID-19 study of adults previously hospitalized for COVID-19 pneumonia. A) Box plots indicate quality of life assessed by SF-36v2 (Quality Metric, https://www.qualitymetric.com). SF-36v2 scores (range 0–100 points) were summarized by using the median for each of the 8 indicated domains. Normal was defined as a score of >50 points for each domain. Median participant scores within each domain are indicated at the top. Horizontal lines within boxes indicate medians; box tops and bottoms indicate upper (third) and lower (first) quartiles; error bars (whiskers) indicate 1.5 times interquartile range. Closed circles indicate outliers. B) Symptoms of study participants assessed by the Modified Living With Pulmonary Fibrosis Assessment Tool. Data are shown as no. (%) participants experiencing each category of symptoms according to 173 enrolled participants. Baseline refers to study baseline (time of enrollment). Questionnaire responses were summarized by using mean patient-reported scores for each symptom domain: dyspnea (7 questions), coughing (5 questions), and fatigue (3 questions). Scores ranged from 0 to 3; the lowest score corresponds to no symptoms and the higher score corresponds to greater symptom severity. SF-36v2, Short Form 36 Question Health Survey version 2.