Volume 31, Number 9—September 2025
Research
Theileria luwenshuni and Novel Babesia spp. Infections in Humans, Yunnan Province, China
Cite This Article
Citation for Media
Abstract
Piroplasmid parasites such as Theileria luwenshuni protozoa pose a global threat to both animal and human health. However, human theileriosis remains underexplored compared to infections caused by Plasmodium and Babesia species parasites. We investigated potential hemoparasite infections among 1,721 persons with fever, anemia, or both in Yunnan Province, China. Molecular detection identified 13 cases positive for T. luwenshuni protozoa, of which 5 patients were further confirmed by Western blot antibody analysis. We also identified 6 babesiosis cases, 3 infections with B. microti and 3 with novel Babesia spp. Subsequent vector and host investigations in the vicinity of the index cases revealed T. luwenshuni protozoa in 1 tick and 53 livestock animals. Of note, 3.3% combined vector-host samples tested positive for genetically diverse Babesia species. Our findings highlight the endemic circulation of T. luwenshuni and Babesia spp. parasites in southwest China, underscoring their importance as emerging public health concerns.
Piroplasmorida, a group of tickborne hemoparasites within the phylum Apicomplexa, includes diverse protozoa species such as Babesia spp. and Theileria spp., which are responsible for causing babesiosis and theileriosis in humans and animals (1,2). Human babesiosis is a globally recognized parasitic zoonosis that primarily targets red blood cells. Parasite transmission to humans occurs predominantly through the bite of an infected tick; however, alternative transmission routes include blood transfusion, perinatal transmission, and organ transplantation (3). Since 1957, the number of human babesiosis cases has increased, posing a growing global public health challenge (4). In China, ≈317 cases of human babesiosis or asymptomatic infections had been reported by 2022 (5). That relatively low number is likely because of underdiagnosis and limited clinical recognition of these protozoan infections. Rare human infections with hemogregarines of the genus Hepatozoon, traditionally considered animal pathogens, have been also detected in immunocompromised patients in Russia, suggesting potential zoonotic spillover of diverse apicomplexan parasites (6).
Theileria spp. parasites primarily infect ruminants such as cattle and sheep, as well as various wild animals (7). Historically, Theileria spp. have not been considered pathogenic to humans. However, a 2014 serosurvey in Italy reported IgG reactivity against T. equi antigens in veterinary practitioners, indicating potential human exposure, particularly among persons at higher risk for infection (8,9). That finding has garnered attention because zoonotic Theileria species were widespread in livestock.
Yunnan Province, located in southwestern China, provides an ideal habitat for various tick species and their host animals because of its distinct geographic features, dense vegetation, and high biodiversity. Those characteristics make the region a hotspot for tickborne diseases (10,11). In Tengchong, along the China–Myanmar border, 8 cases of human babesiosis caused by Babesia microti and 2 case co-infections with B. microti and Plasmodium parasites were confirmed in 2013 (12). To enhance understanding of the prevalence of tickborne protozoa in China, we studied patients experiencing fever and anemia in Yunnan Province and traced potential sources of infection by examining protozoan prevalence in domestic animals, small wild animals, and ticks within the affected areas. Our goal was to further characterize the epidemiologic and clinical features of piroplasmid parasite infections and identified their possible sources.
Identification of Piroplasmosis in Patients
We conducted a retrospective investigation among participants experiencing unexplained fever or anemia across 10 counties in Yunnan Province, China, during May 2017–June 2020. Demographic data, medical history, and epidemiologic exposure history had been collected through a structured questionnaire. We retrieved data on clinical manifestations, underlying medical conditions, laboratory test results, treatments, and outcomes from medical records; 2 investigators cross-validated the data. The patients’ blood samples were collected at various time points during their hospital stay; a portion of each patient’s blood samples were immediately processed for blood smear preparation, and the residual blood was stored at −80°C until nucleic acid extraction for batch PCR amplification and downstream analysis. The Ethics Committee of the Yunnan Provincial Institute of Endemic Disease Control approved the study (approval no. 2016-005), which was conducted in accordance with medical research regulations in China. All participants provided written informed consent before their inclusion in the study.
Source Tracing Investigation
As part of the study, we conducted retrospective testing on ticks, livestock, and small mammals from areas surrounding the participants. Wild small mammals were captured using snap traps, and aseptic tissue samples, including liver and spleen, were collected and stored at −80°C for subsequent analysis. We taxonomically identified the captured animals to the species level on the basis of external morphology, coloration, measurements, and dental characteristics. We collected whole-blood samples from livestock via jugular vein puncture using EDTA anticoagulant tubes and stored them at −20°C until DNA extraction. We manually removed ticks from livestock and collected host-seeking ticks by flag-sweeping vegetation at the same sampling sites. An entomologist identified tick species. We preserved tick samples at −80°C before DNA extraction.
PCR Amplification and Sequencing
We extracted DNA from human blood, livestock blood, small mammal tissues, and tick samples using the DNeasy Blood & Tissue Kit (QIAGEN, https://www.qiagen.com) in accordance with manufacturer’s instructions. To detect Theileria and Babesia parasites, we performed nested PCR targeting the 18S rRNA gene using outer primers Piro0F/Piro6R and inner primers Piro1F/Piro5.5R (13,14), followed by agarose gel electrophoresis and Sanger sequencing. We amplified additional genetic loci, including the 5.8S rRNA (303 bp), internal transcribed spacer region (1,300 bp), P32 immunodominant protein gene (875 bp), and cytochrome oxidase subunit I (1,200 bp). We conducted concurrent testing for other potential infections with Rickettsia spp., Borrelia burgdorferi, B. recurrentis, and Bartonella spp., which could potentially be transmitted by similar transmission routes or cause similar symptoms, to exclude differential diagnoses (Appendix 1 Table 1).
Phylogenetic Analysis
We performed phylogenetic analysis using sequences assembled with the CLC Main Workbench version5.5 (QIAGEN). We conducted comparative analysis against sequences in GenBank using BLAST (https://blast.ncbi.nlm.nih.gov). We conducted phylogenetic analysis of all sequences using MEGA version 11.0 software (https://www.megasoftware.net). We constructed phylogenetic trees using the neighbor-joining method with the p-distance model based on 1,000 bootstrap replicates.
Morphologic and Serologic Testing
We stained thin peripheral blood smears collected from the participants with Giemsa and examined under a light microscope (Olympus, https://evidentscientific.com). We used the recombinant T. uilenbergi immunodominant protein (rTuIP) as the diagnostic antigen for Western blot analysis (15,16). Anhui Global Gene Technology Company (Chuzhou, China) conducted protein expression and purification using the pQE31 vector as the expression vector and BL21 (DE3) as the expression host. Tengchong People’s Hospital (Tengchong County, China) provided serum samples collected for rTuIP antibody detection from some patients with suspected protozoan infections and used serum samples from healthy persons as negative controls.
We separated recombinantly expressed TuIP protein (20 μg) using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred it onto a polyvinylidene difluoride membrane (0.45 μm pore size). To block nonspecific binding, we incubated the membrane with 5% skimmed milk powder in Tris-buffered saline containing Tween 20 for 2 hours. We used patient serum samples diluted at various ratios (1:25–1:800) as primary antibodies; serum samples from healthy persons diluted at 1:100 served as negative controls. We then incubated the membrane overnight at 4°C. We applied horseradish peroxidase–labeled human IgG secondary antibody, diluted at 1:5,000, for a 1-hour incubation at room temperature. After washes in Tris-buffered saline containing Tween 20, we detected the signal using an enhanced chemiluminescence developing reagent.
Identification of Piroplasmosis in Participants
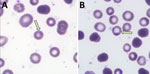
We screened a total of 1,721 participants from 10 counties in Yunnan Province for protozoan infections; 1,362 participants were experiencing fever, and 359 had anemia, (Appendix 1 Table 2). Among those, we identified 18 inpatients and 1 outpatient to have suspected protozoan infections by 18S rRNA sequencing of blood samples; amplification for additional T. luwenshuni genetic loci was not successful. Meanwhile, molecular testing for Rickettsia spp., B. burgdorferi sensu lato, B. recurrentis, and Bartonella spp. yielded uniformly negative results. We examined peripheral blood smears from the 19 suspected cases for intraerythrocytic parasites using Giemsa staining; patient 2 tested positive for piroplasmosis parasites (Figure 1). No parasites were detected in the blood smears of the remaining patients.
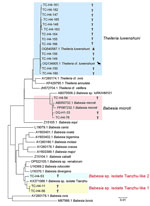
We successfully amplified and sequenced the 18S rRNA gene of T. luwenshuni parasites from 13 blood samples, yielding sequences ≈1,600 bp long. Those sequences exhibited a similarity range of 99.28%–100% among the samples; we deposited them into GenBank (accession nos. PQ720759–71). Similarity analysis indicated that the T. luwenshuni samples shared 99.69% identity with a reference sequence isolated from a Haemaphysalis longicornis tick (GenBank accession no. OQ540587.1) and 98.60% identity with a T. luwenshuni isolate from goats (GenBank accession no. OQ134905.1), both collected in Shandong Province, China. Phylogenetic analysis further showed that the sequences clustered within the same monophyletic branch (Figure 2; Appendix 1 Table 3).
We successfully amplified and sequenced the 18S rRNA gene of Babesia spp. from 6 blood samples, yielding sequences ≈1,600 bp long. We submitted those sequences to GenBank (accession nos. PQ720772–5, PQ722837, PQ722838). Phylogenetic analysis demonstrated that samples DQ-H1-33, TC-H4-76, and TC-H4-54 clustered with multiple B. microti strains from reference sequences (GenBank accession nos. KF410825.1 and AB050732.1), exhibiting a high genetic similarity (Figure 2). In addition, samples TC-H4-11 and TC-H4-56 clustered with Babesia Tianzhu–like subgroups that were identified in white yaks in China (14). Meanwhile, sample TC-H4-53 clustered with another subgroup, which suggested that TC-H4-53 may represent an uncharacterized or novel Babesia variant or strain (Figure 2).
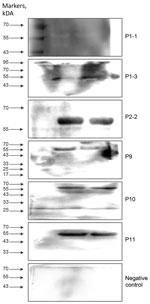
Western blot analysis confirmed the diagnostic utility of the rTuIP protein for detecting T. luwenshuni infections. Of 11 serum samples from participants with suspected hemoprotozoan infections, 6 samples (from 5 patients) exhibited characteristic rTuIP-specific bands at a 1:25 dilution (Figure 3). Of note, longitudinal samples from patient 1 (P1-1 and P1-3) both exhibited positive reactivity. Control samples from healthy participants showed no target protein–specific bands.
Analysis of longitudinal antibody responses also revealed dynamic seroconversion patterns over time (Appendix 2 Figure, panel A). Sample P1-1 exhibited faint reactivity at 1:25 dilution but was negative at >1:50. In contrast, subsequent samples from the same patient (P1-2 and P1-3) displayed strong immunoreactivity across all consecutive doubling tested dilutions (1:50–1:800) (Appendix 2 Figure, panel A), indicating the maturation of antibody titers. We saw similar consistently high-titer responses in patient 2 at 2 timepoints, with clear bands visible from 1:50–1:800 dilutions (Appendix 2 Figure, panel B).
Epidemiologic and Clinical Characteristics of the Patients
Among the 13 patients with suspected T. luwenshuni infection (Appendix 1 Table 4), the median age was 40 (interquartile range [IQR] 32.5–68.0) years; 10 (77%) patients were male and 13 (23%) female. The median hospital stay was 13 (IQR 7.5–17.0) days. All patients were engaged in farming. Underlying conditions noted at admission included renal failure in 5 (38%) patients, upper gastrointestinal bleeding in 5 (38%) patients, and a history of cancer in 3 (23%) patients; 1 patient reported prior blood transfusion history. The primary clinical manifestations were anorexia (8 [62%] patients), listlessness (8 [62%] patients), malaise (5 [38%] patients), and melena (4 [31%] patients). Other reported symptoms included vomiting (4 [31%] patients), dizziness (3 [23%] patients), and palpitations (3 [23%] patients); cough, jaundice, splenomegaly, and weight loss were each observed in 1 patient (8%). One patient reported neurologic symptoms (Appendix 1 Table 4). Laboratory findings revealed anemia in all 13 patients and leukocytosis in 2 (15%) patients. Elevated serum levels of aspartate aminotransferase, alanine aminotransferase, or gamma-glutamyl transferase were present in 1 patient (8%). All patients received general supportive and symptomatic treatment; 4 (31%) patients underwent blood transfusions, and 5 (38%) patients required hemodialysis (Table 1).
Among the 5 patients with suspected Babesia infections (Appendix 1 Table 5), median age was 56 years (IQR 25.5–59.5 years); 4 (80%) patients were male and 1 (20%) female. Similar to the T. luwenshuni cohort, all patients were engaged in farming. Underlying conditions were renal failure in 1 patient (20%) and a history of upper gastrointestinal bleeding in 3 patients (60%). Median hospitalization duration was relatively short at 10 days (IQR 5.5–10.5 days). Two patients (40%) had a history of blood transfusion; 1 (20%) patient had a history of gastric hemorrhage or hypertension. The most frequently reported symptoms were nonspecific. Four (80%) patients experienced dizziness and malaise, 3 (60%) patients melena, and 2 (40%) listlessness. Bloating, chest discomfort, palpitations, and dyspnea were all present in 1 (20%) patient. Of note, none of the patients in this group reported vomiting, jaundice, or splenomegaly. Laboratory tests revealed anemia in all patients and leucopenia in 1 (20%) patient. Elevated serum levels of aspartate aminotransferase, alanine aminotransferase, or gamma-glutamyl transferase were not detected in any case. In terms of treatment, 3 (60%) patients received blood transfusions, and 1 patient (20%) required hemodialysis (Table 1).
Traceability Survey
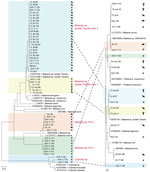
We performed PCR analyses on 2,100 livestock, 1,530 wild small mammals, and 1,516 ticks collected from 10 counties in Yunnan Province to detect Piroplasmorida infections (Table 2). We used the same protocol previously optimized for human diagnostics. Among the livestock samples, 147 (7.00%) tested positive. We constructed a phylogenetic tree using 18S rRNA sequences (≈1,600 bp) to classify the positive samples into 5 species: B. microti, Babesia sp. Tianzhu-like, B. bigemina, B. bovis, and T. luwenshuni (Figures 4, 5). We detected 1 strain of B. microti and 2 strains of T. luwenshuni parasites in livestock from Tengchong County. The overall positivity rate for small mammals was 2.42%. We identified B. microti as the predominant species. Phylogenetic analysis identified a variant, tentatively named Babesia sp. YN-2, in small mammals (Figure 4). Among tick samples, 37 (2.44%) tested positive for Piroplasmorida; detected species including B. microti, B. bigemina, Babesia sp. Tianzhu-like, B. bovis, Babesia sp., Colpoda sp., and T. luwenshuni. Phylogenetic analysis further classified the novel Babesia sp. into 2 clades: Babesia sp. YN-2 and Babesia sp. YN-3.
Genetic analysis of the Babesia sp. Tianzhu-like isolates identified in patients, livestock, and ticks yielded 2 major clades with high homology (96.11%–98.67%) but distinct variations of 21–62 bp. We tentatively designated those clades as Babesia sp. isolate Tianzhu-like 1 and Babesia sp. isolate Tianzhu-like 2. The phylogenetic tree depicted the relationships among Babesia and Theileria species collected from various hosts and vectors. Of note, we identified B. microti in multiple host and vector types, indicating its high adaptability across diverse environments (Figure 5). In addition, we detected the novel Babesia sp. YN-2 in both small mammals and ticks, suggesting the existence of a potential transmission cycle between wildlife and arthropod vectors. The detection of Babesia sp. isolate Tianzhu-like parasites across humans, livestock, and ticks highlights its broad host range and geographic distribution. Similarly, the widespread occurrence of T. luwenshuni parasites in humans, livestock, and ticks underscores the species’ potential for transmission among multiple hosts.
This study identified 4 tickborne hemoparasites with human infectivity, including a zoonotic infection caused by 2 known pathogenic agents and 2 novel Babesia species. It characterized the diversity and complexity of tickborne protozoa in ticks, small wild animal hosts, and livestock in southwestern China. Our findings are critical for public health and the enhancement of parasitic disease surveillance systems in Yunnan Province. The study presented a clinically relevant finding of human Theileria infections, corroborated through molecular diagnostics and Western blot serology, which were validated previously (16) and were further confirmed by our serologic titers and longitudinal testing, with no cross-reactivity against other tickborne pathogens.
A previous serosurvey in Italy (8) reported IgG reactivity against T. equi antigens in veterinary practitioners, indicating potential human exposure to this pathogen, particularly among persons at heightened risk for infection. That result raised considerable interest because of the prevalence of zoonotic Theileria species in livestock. Of note, our study reported a series of human infections with T. luwenshuni parasites, previously recognized as primarily affecting ruminants, with less reported pathogenicity in humans.
Our study also identified potential host-vector transmission routes involving domestic animals and ticks. Genetic analysis indicated that T. luwenshuni isolates exhibited high similarity to vector-derived strains from Shandong Province, in the east of China. That finding indicates a widespread distribution, an emerging epidemic trend, and the potential for cross-species transmission of these organisms. Those results provide valuable insights for future epidemiologic investigations targeting affected populations, hosts, and vectors across diverse regions.
In this study, we detected >6 species of Piroplasmorida in livestock, small mammals, and ticks: B. microti, Babesia sp. Tianzhu-like, B. bigemina, B. bovis, Babesia sp., and T. luwenshuni. We observed genetic diversity within Babesia sp. Tianzhu-like; homology was 96.11%–98.67% and 21–62 bp variations. Four of those species are known or suspected to exhibit explicit pathogenicity. Of note, we identified Babesia sp. Tianzhu-like 1 in patients, livestock, and ticks, with homology of 99.10% –99.61%. However, the exact infection source remains uncertain for some patients who lacked a clear history of tick bites but reported previous blood transfusions. In addition, most patients were immunocompromised, raising questions about the potential for opportunistic infections. Similar to B. venatorum and B. microti (17), asymptomatic carriage of T. luwenshuni and other Babesia spp. parasites may occur in healthy persons. Furthermore, as the endemic regions expand, T. luwenshuni parasites may emerge as a more frequent complication in immunosuppressed hosts, akin to patterns observed in human babesiosis (18). Enhanced surveillance, particularly among blood donors in this region, is imperative (19).
Since the earliest report of Theileria parasites in Sichuan Province in 1958, >4 species have been documented in China: T. luwenshuni, T. unilenbergi, T. ovis, and T. annulata. Among them, T. luwenshuni, designated in 2007 (20,21), has been a species recognized for its high pathogenicity in sheep and goats. The presence of T. luwenshuni parasites has now been confirmed in multiple provinces, including Yunnan, Hubei, Henan, Gansu, Jilin, Hunan, and Shandong (20). Of note, the prevalence of T. luwenshuni parasites in goats in Shandong Province has been reported to reach 81.5%, higher than rates previously reported in small ruminants and deer in central and northwestern China (22–25). Those findings suggest an emerging epidemic trend of T. luwenshuni infections in humans across multiple regions.
B. microti is the predominant species causing human babesiosis in the United States. In China, it has been associated with >100 reported human infections, most occurring in Guangxi Province (9). Of note, 10 B. microti infections were identified in a study of 449 patients with fever in Tengchong County, Yunnan Province, along the China–Myanmar border (12). Babesia sp. isolate Tianzhu was initially discovered in Tianzhu Tibetan Autonomous County, northwestern China, in 2017. In our study, we identified this agent in both ticks and water buffalo, demonstrating a close genetic relationship to isolates obtained from 2 human patients (Figure 2); that finding suggests that Babesia sp. isolate Tianzhu may present a public health risk.
A limitation of this study is that T. luwenshuni infections were identified in immunocompromised patients, and as a retrospective investigation, it was not possible to establish causal relationships between the protozoan infection and patient symptoms or clinical features. Specifically, whether the observed anemia resulted directly from protozoan-induced red blood cell damage or from comorbid conditions (e.g., bleeding disorders or renal failure) remains unclear. Future research should prioritize prospective cohort studies at in sentinel hospitals involving similar patients. Such studies should exclude anemia caused by renal failure or other underlying conditions using interventions such as erythropoietin combined with iron therapy. Those patients should receive antiprotozoal therapy with monitoring of parameters, such as reticulocyte counts and L-lactic dehydrogenase levels, to assess treatment efficacy.
In conclusion, we used molecular biology techniques, including PCR, serology, and phylogenetic analysis, to characterize the diversity and potential risks of tickborne protozoa in Yunnan Province, China. Our findings confirmed the endemic circulation of T. luwenshuni and multiple Babesia spp. parasites in southwestern China. Further investigation of T. luwenshuni infection will elucidate transmission dynamics, clinical impact, and targeted prevention strategies, as well as its implications for public health. Clinicians in this region should remain aware of these emerging public health concerns.
Mrs. Xiang is a DPH candidate in State Key Laboratory of Pathogen and Biosecurity, Academy of Military Medical Sciences, Beijing. Her primary research interest is the prevention and control of vectorborne pathogens.
Acknowledgment
The Natural Science Foundation of China supported this study (grant no. U2002219 to J.-F. J.).
References
- Almazán C, Scimeca RC, Reichard MV, Mosqueda J. Babesiosis and Theileriosis in North America. Pathogens. 2022;11:168. DOIPubMedGoogle Scholar
- Karasartova D, Gureser AS, Gokce T, Celebi B, Yapar D, Keskin A, et al. Bacterial and protozoal pathogens found in ticks collected from humans in Corum province of Turkey. PLoS Negl Trop Dis. 2018;12:
e0006395 . DOIPubMedGoogle Scholar - Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012;12:1788–809. DOIPubMedGoogle Scholar
- Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9:11–6.PubMedGoogle Scholar
- Chen MX, Xue JB, Ai L, Song P, Cai YC, Chen JX. Epidemic status and research progress of babesiosis in China [in Chinese]. J Trop Dis Parasitol. 2022;20:149–57. DOIGoogle Scholar
- Shuĭkina EE, Beĭer TV, Sergiev VP, Iastrebova RI. [Detection of hemogregarin of the genus Hepatozoon in patients in Russia] [in Russian]. Med Parazitol (Mosk). 2004; (
4 ):3–6.PubMedGoogle Scholar - Sivakumar T, Hayashida K, Sugimoto C, Yokoyama N. Evolution and genetic diversity of Theileria. Infect Genet Evol. 2014;27:250–63. DOIPubMedGoogle Scholar
- Gabrielli S, Calderini P, Cassini R, Galuppi R, Tampieri MP, Pietrobelli M, et al. Human exposure to piroplasms in Central and Northern Italy. Vet Ital. 2014;50:41–7. DOIPubMedGoogle Scholar
- Chen Z, Li H, Gao X, Bian A, Yan H, Kong D, et al. Human Babesiosis in China: a systematic review. Parasitol Res. 2019;118:1103–12. DOIPubMedGoogle Scholar
- Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E. Emerging tick-borne diseases. Clin Microbiol Rev. 2020;33:e00083–18. DOIPubMedGoogle Scholar
- Zhao GP, Wang YX, Fan ZW, Ji Y, Liu MJ, Zhang WH, et al. Mapping ticks and tick-borne pathogens in China. Nat Commun. 2021;12:1075. DOIPubMedGoogle Scholar
- Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, et al. Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty. 2013;2:24. DOIPubMedGoogle Scholar
- Kawabuchi T, Tsuji M, Sado A, Matoba Y, Asakawa M, Ishihara C. Babesia microti-like parasites detected in feral raccoons (Procyon lotor) captured in Hokkaido, Japan. J Vet Med Sci. 2005;67:825–7. DOIPubMedGoogle Scholar
- Liu J, Guan G, Li Y, Liu A, Luo J, Yin H. A molecular survey of Babesia species and detection of a new Babesia species by DNA related to B. venatorum from white yaks in Tianzhu, China. Front Microbiol. 2017;8:419. DOIPubMedGoogle Scholar
- Liu Z, Li Y, Salih DE, Luo J, Ahmed JS, Seitzer U, et al. Validation of a recombinant protein indirect ELISA for the detection of specific antibodies against Theileria uilenbergi and Theileria luwenshuni in small ruminants. Vet Parasitol. 2014;204:139–45. DOIPubMedGoogle Scholar
- Liu Z, Wang Z, Yin H, Luo J, Zhang B, Kullmann B, et al. Identification of Theileria uilenbergi immunodominant protein for development of an indirect ELISA for diagnosis of ovine theileriosis. Int J Parasitol. 2010;40:591–8. DOIPubMedGoogle Scholar
- Ruebush TK II, Juranek DD, Chisholm ES, Snow PC, Healy GR, Sulzer AJ. Human babesiosis on Nantucket Island. Evidence for self-limited and subclinical infections. N Engl J Med. 1977;297:825–7. DOIPubMedGoogle Scholar
- Heller HM. Babesiosis in immunosuppressed hosts: pathogenesis, diagnosis and management. Curr Opin Infect Dis. 2024;37:327–32. DOIPubMedGoogle Scholar
- Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15:196–203. DOIPubMedGoogle Scholar
- Yin H, Liu Z, Guan G, Liu A, Ma M, Ren Q, et al. Detection and differentiation of Theileria luwenshuni and T. uilenbergi infection in small ruminants by PCR. Transbound Emerg Dis. 2008;55:233–7. DOIPubMedGoogle Scholar
- Yin H, Schnittger L, Luo J, Seitzer U, Ahmed JS. Ovine theileriosis in China: a new look at an old story. Parasitol Res. 2007;101(Suppl 2):S191–5. DOIPubMedGoogle Scholar
- Wang BH, Du LF, Zhang MZ, Xia LY, Li C, Lin ZT, et al. Genomic characterization of Theileria luwenshuni strain Cheeloo. Microbiol Spectr. 2023;11:
e0030123 . DOIPubMedGoogle Scholar - Li Y, Chen Z, Liu Z, Liu J, Yang J, Li Q, et al. Molecular identification of Theileria parasites of northwestern Chinese Cervidae. Parasit Vectors. 2014;7:225. DOIPubMedGoogle Scholar
- Li Y, Zhang X, Liu Z, Chen Z, Yang J, He H, et al. An epidemiological survey of Theileria infections in small ruminants in central China. Vet Parasitol. 2014;200:198–202. DOIPubMedGoogle Scholar
- Zhang X, Liu Z, Yang J, Chen Z, Guan G, Ren Q, et al. Multiplex PCR for diagnosis of Theileria uilenbergi, Theileria luwenshuni, and Theileria ovis in small ruminants. Parasitol Res. 2014;113:527–31. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: August 18, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence: Jia-Fu Jiang and Yi Sun, State Key Laboratory of Pathogen and Biosecurity, Academy of Military Medical Sciences, 20 Dong-Da St, Fengtai District, Beijing 100071, China
Top