Volume 13, Number 9—September 2007
Dispatch
Anaplasma platys in Dogs, Chile
Abstract
We conducted a 16S rRNA nested PCR for the genus Ehrlichia and Ehrlichia spp. with blood samples from 30 ill dogs in Chile. Phylogenetic analysis was performed by using groESL gene amplification. We identified Anaplasma platys as 1 of the etiologic agents of canine ehrlichiosis.
Ehrlichioses are recognized as important emerging tickborne diseases in humans and wild and domestic animals. The brown dog tick, Rhipicephalus sanguineus, is the main tick that infests dogs in Chile (1). This tick species is a vector of Ehrlichia canis and has been implicated, but not confirmed, as a vector of Anaplasma platys (2). Serologic and clinical evidence of canine ehrlichiosis and serologic evidence of human ehrlichiosis have been reported in Chile (3,4). The purpose of this study was to identify the etiologic agent of canine ehrlichiosis in Chile.
Blood samples were obtained from 30 pet dogs seen in a private veterinary clinic in Santiago, Chile, with tick infestation and clinical signs compatible with ehrlichiosis (hemorrhagic manifestations and thrombocytopenia). We performed a nested PCR to amplify a portion of the 16S rRNA gene by using specific primers for the genus Ehrlichia and for Ehrlichia spp. DNA was extracted from 300 μL of whole blood by using the Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA). For Ehrlichia genus–specific PCR, 2.5 μL of DNA was amplified by using outer primers EHR-OUT1 and EHR-OUT2 and inner primers GE2F and EHRL3-IP2 in 1 reaction with a final volume of 25 μL (5) (Table 1).
The first-round amplification included 20 cycles of denaturation at 94°C for 45 s, annealing at 72°C for 1.5 min, and chain extension at 72°C for 1.5 min. The second-round amplification included 50 cycles of denaturation at 94°C for 45 s, annealing at 50°C for 1 min, and chain extension at 72°C for 1 min, followed by a final extension at 72°C for 5 min. Amplification products were analyzed by agarose gel electrophoresis. The expected size of the amplification product was 120 bp. A. phagocytophilum DNA was used as a positive control (provided by Didier Raoult). For Ehrlichia spp.–specific amplification, we used the same set of outer primers for Anaplasmataceae and specific inner primers for A. phagocytophilum (6), E. chaffeensis, E. ewingii, and E. canis (5) (Table 1). For A. platys amplification, we used inner primers developed by Kordick et al. (EHRL3-IP2–E. platys) (7) (Table 1). Expected sizes of amplification products were 546, 395, 395, 389, and 151 bp, respectively.
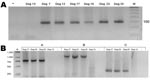
The Ehrlichia genus PCR resulted in the expected DNA band in 6 of 30 dogs (dogs 7, 12, 17, 19, 23, and 25). These 6 samples were positive only for A. platys, showing the expected 151-bp product, and negative for other species tested (Figure 1, panel A). A. platys PCR was also conducted on the remaining 24 Ehrlichia-negative samples; none were positive.
DNA obtained from 3 16S rRNA PCR products (dogs 7, 17, and 25) was purified by using a commercial kit (Rapid Gel Extraction System; Marligen Biosciences, Ljamsville, Germany) and sequenced twice with an ABI 3100 genetic analyzer (Model 3100; Applied Biosystems, Foster City, CA, USA). The 16S rRNA sequences obtained were compared by using BLAST (www.ncbi.nlm.nih.gov/blast) with sequences available at GenBank. Sequences obtained were similar to that of A. platys strain Okinawa 1 (GenBank accession no. AF536828), with similarities of 98%, 95%, and 98%, respectively. GenBank accession nos. for 16S rRNA sequences of A. platys strains obtained in this study are DQ125260 and DQ125261, which correspond to strains from dogs 7 and 17, respectively.
For phylogenetic analysis, the groESL gene of A. platys was amplified from samples positive for A. platys 16S rRNA that had sufficient amounts of DNA (dogs 17, 23, and 25) and from 1 negative sample (dog 13). Reactions contained 2 μL of purified DNA as template in a total volume of 25 μL. Amplifications contained 1.25 U Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), 3 mmol/L MgCl2, 2.5 mmol/L deoxynucleotide triphosphates (Invitrogen), and 0.2 pmol/L of primers EEgro1F and EEgro2R (8) (Table 1). DNA was denatured by heating at 95°C for 10 min. PCR amplification included 40 cycles of denaturation at 95°C for 1.5 min, annealing at 52°C for 2 min, and extension at 72°C for 1.5 min, followed by a final extension at 72°C for 10 min. For nested amplifications, 1 μL of primary PCR products was used as the template in a total volume of 25 μL. Reaction conditions were the same as for primary amplifications. The primers used were SQ3F, SQ5F, SQ4R, and SQ6R (9) (Table 1). PCR products were analyzed by 1.5% agarose gel electrophoresis.
We amplified 3 overlapping fragments (790, 1,170, and 360 bp) in 3 16S rRNA–positive samples (Figure 1, panel B). These DNAs were purified by using a commercial kit (Rapid Gel Extraction System; Marligen), sequenced, and analyzed for phylogenetic relationships. Multiple alignment analysis was performed with the ClustalW program (www.ebi.ac.uk/clustalw). Calculation of distance matrices and construction of a phylogenetic tree were made with MEGA 3.1 software (www.megasoftware.net). A phylogenetic tree was constructed by the neighbor-joining method and distance matrices for the aligned sequences were calculated by using the Kimura 2-parameter method. Stability of the tree was estimated by bootstrap analysis of 1,000 replications. A final sequence of 686 bp obtained from the overlapping fragments was used for comparison and showed 100% identity between the 3 Chilean sequences and 99.8% similarity with sequences of the A. platys groESL gene deposited in GenBank (Table 2). Phylogenetic relationships of Chilean A. platys strains with other Anaplasmataceae species are shown in Figure 2. GenBank accession no. for the groESL gene sequence of A. platys is EF201806 (corresponding to dogs 17, 23, and 25).
We identified A. platys DNA in the blood of 6 dogs with clinical signs indicative of ehrlichiosis. These findings support the conclusion that A. platys is an etiologic agent of canine ehrlichiosis in Chile.
Since its first report in the United States in 1978 (10), A. platys has been described in several countries as the etiologic agent of cyclic thrombocytopenia in dogs. A tick vector of A. platys has not been determined, although R. sanguineus is the most suspected species (2). Because R. sanguineus is the only tick species that infests dogs in Santiago (1), our results support the conclusion that this species is the vector of A. platys in Chile.
A wide range of clinical manifestations of canine cyclic thrombocytopenia has been described. Cases from the United States have been described as mild or asymptomatic (10), and cases from Spain have more severe symptoms (11), which also seems to be the case in Chile. This variability in clinical symptoms of infection has not been clearly associated with strain variations (11–13).
Low diversity was observed when groESL gene sequences of Chilean strains were compared with other A. platys strains available in GenBank. This finding has also been observed in strains from different geographic origins (13). Recent studies have shown more genetic variability when sequences of the gltA gene were used (11,12).
Evidence of the zoonotic potential of A. platys is scarce. In Venezuela, a few symptomatic human cases have been diagnosed since 1992 by the presence of platelet morulae in blood smears (14). Monocytic and platelet morulae were reported in a 17-month-old girl with fever and rash (15). However, none of these cases have been confirmed by molecular assays. Further studies that investigate the pathogenic and zoonotic role of A. platys should be conducted.
Dr Abarca is a pediatrician and infectious disease specialist and associate professor of pediatrics at the Pontificia Universidad Católica de Chile School of Medicine. Her primary research interests include emerging infectious diseases, zoonotic diseases, pet infections, and pediatric vaccinology.
Acknowledgments
We thank Marcelo Labruna for critical comments on the manuscript.
This study was supported by a grant from Universidad Santo Tomás, Chile.
References
- González-Acuña D, Guglielmone AA. Ticks (Acari: Ixodoidea: Argasidae, Ixodidae) of Chile. Exp Appl Acarol. 2005;35:147–63. DOIPubMedGoogle Scholar
- Sanogo YO, Davoust B, Inokuma H, Camicas JL, Parola P, Brouqui P. First evidence of Anaplasma platys in Rhipicephalus sanguineus (Acari: Ixodida) collected from dogs in Africa. Onderstepoort J Vet Res. 2003;70:205–12.PubMedGoogle Scholar
- López J, Castillo A, Muñoz M, Hildebrand S. Hallazgo de Ehrlichia canis en Chile, informe preliminar. Archivos de Medicina Veterinaria. 1999;31:211–4. DOIGoogle Scholar
- López J, Rivera M, Concha JC, Gatica S, Loeffeholz M, Barriga O. Serologic evidence for human ehrlichiosis in Chile. Rev Med Chil. 2003;131:67–70.PubMedGoogle Scholar
- Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645–51.PubMedGoogle Scholar
- Massung RF, Slater K, Owens J, Nicholson W, Mather T, Solberg V, Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–5.PubMedGoogle Scholar
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–8.PubMedGoogle Scholar
- Inokuma H, Fujii K, Okuda M, Onishi T, Beaufils JP, Raoult D, Determination of the nucleotide sequences of heat shock operon groESL and the citrate synthase gene (gltA) of Anaplasma (Ehrlichia) platys for phylogenetic and diagnostic studies. Clin Diagn Lab Immunol. 2002;9:1132–6.PubMedGoogle Scholar
- Chae JS, Foley JE, Dumler JS, Madigan JE. Comparison of the nucleotide sequences of 16S rRNA, 444 Ep-ank, and groESL heat shock operon genes in naturally occurring Ehrlichia equi and human granulocytic ehrlichiosis agent isolates from northern California. J Clin Microbiol. 2000;38:1364–9.PubMedGoogle Scholar
- Harvey JW, Simpson CF, Gaskin JM. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J Infect Dis. 1978;137:182–8.PubMedGoogle Scholar
- Aguirre E, Tesouro MA, Ruiz L, Amusategui I, Sainz A. Genetic characterization of Anaplasma (Ehrlichia) platys in dogs in Spain. J Vet Med B Infect Dis Vet Public Health. 2006;53:197–200. DOIPubMedGoogle Scholar
- de la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet Res. 2006;2:24. DOIPubMedGoogle Scholar
- Huang H, Unver A, Pérez MJ, Orellana NG, Rikihisa Y. Prevalence and molecular analysis of Anaplasma platys in dogs in Lara. Venezuela. Braz J Microbiol. 2005;36:211–6. DOIGoogle Scholar
- Arraga-Alvarado C, Palmar M, Parra O, Salas P. Fine structural characterisation of a Rickettsia-like organism in human platelets from patients with symptoms of ehrlichiosis. J Med Microbiol. 1999;48:991–7. DOIPubMedGoogle Scholar
- Arraga-Alvarado C, Montero-Ojeda M, Bernardoni A, Anderson BE, Parra O. Human ehrlichiosis: report of the 1st case in Venezuela. Invest Clin. 1996;37:35–49.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 13, Number 9—September 2007
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Katia Abarca, Infectious Diseases and Molecular Virology Laboratory, Marcoleta 391, Third Floor, Pontificia Universidad Católica de Chile, Santiago, Chile;
Top