Volume 17, Number 9—September 2011
Research
Classical Bovine Spongiform Encephalopathy by Transmission of H-Type Prion in Homologous Prion Protein Context
Figure 3
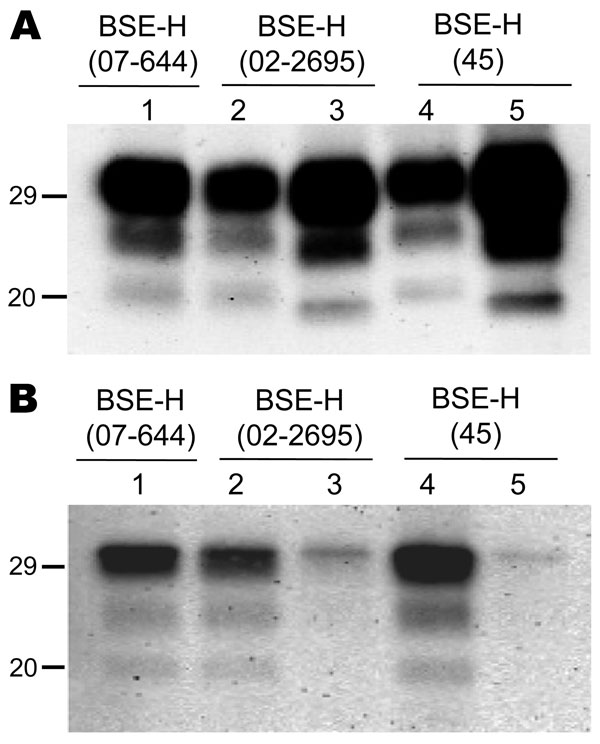
Figure 3. Comparative Western blot analyses with Sha31 and 12B2 monoclonal antibodies (mAbs) of brain protease-resistant prion protein (PrPres) from BSE-H–infected mice. Mice infected with isolate 07-644 (lane 1), 02-2695 (lanes 2 and 3), or 45 (lanes 4 and 5) at first passage showing either high-type (lanes 1, 2, and 4) or classical BSE–like PrPres molecular profile (lanes 3 and 5). Panel A was shown with Sha31 mAb; panel B was shown with 12B2 mAb. The same quantities of PrPres were loaded in both panels A and B. Values to the left indicate molecular mass in kDa. BSE, bovine spongiform encephalopathy; BSE-H, unglycosylated PrPres that is higher than classical BSE.
Page created: September 06, 2011
Page updated: September 06, 2011
Page reviewed: September 06, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.