Volume 17, Number 9—September 2011
Research
Classical Bovine Spongiform Encephalopathy by Transmission of H-Type Prion in Homologous Prion Protein Context
Figure 7
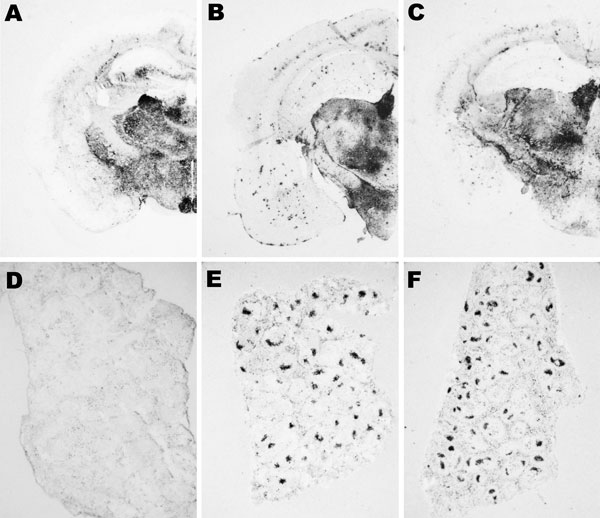
Figure 7. Abnormal isoform of host-encoded prion protein (PrPSc) deposition patterns in brain and spleen from Tg110 mice infected with BSE-H. A–C) Paraffin-embedded tissue (PET) blots of representative coronal sections at the level of the hippocampus from Tg110 mice infected with atypical BSE-H (isolate 02-2695, first passage) showing either high-type (A) or classical-type protease-resistant prion protein (PrPres) phenotype (B). PET blot from Tg110 mice infected with BSE-C (C) is included for comparison. D–F) PET blots of representative spleen sections: Tg110 mice infected with atypical BSE-H (isolate 02-2695) showing high-type PrPres in the brain were consistently scored as negative for PrPSc detection (D), whereas clear PrPSc deposits were always detected in BSE-H infected mice exhibiting BSE-C–like features (E), as in mice infected with BSE-C (F). Original magnification levels: panels A–C, ×20; panels D–F, ×6.BSE, bovine spongiform encephalopathy; BSE-H, unglycosylated PrPres that is higher than BSE-C; BSE-C, classical BSE.