Volume 19, Number 2—February 2013
Dispatch
Human Granulocytic Anaplasmosis, Japan
Cite This Article
Citation for Media
Abstract
We retrospectively confirmed 2 cases of human Anaplasma phagocytophilum infection. Patient blood samples contained unique p44/msp2 for the pathogen, and antibodies bound to A. phagocytophilum antigens propagated in THP-1 rather than HL60 cells. Unless both cell lines are used for serodiagnosis of rickettsiosis-like infections, cases of human granulocytic anaplasmosis could go undetected.
Japanese spotted fever (JSF) and scrub typhus, which are caused by infection with Rickettsia japonica and Orientia tsutsugamushi, respectively, are common rickettsioses in Japan (1). National surveillance (http://idsc.nih.go.jp/idwr/CDROM/Main.html [in Japanese]) indicates that JSF occurs frequently in central and western Japan and that scrub typhus is present throughout Japan, except in Hokkaido. In JSF- and scrub typhus–endemic areas, cases of non-JSP and non–scrub typhus disease with rickettsiosis-like fever have often been reported. And, human infection with R. heilongjiangensis, a spotted fever group (SFG) rickettsia, has been identified in Japan (2). Furthermore, Anaplasma phagocytophilum has been detected in Ixodes persulcatus and I. ovatus ticks, and Ehrlichia chaffeensis has been detected in deer (3–6). More recently, we identified A. phagocytophilum infection in ticks (Haemaphysalis formosensis, H. longicornis, H. megaspinosa, and Amblyomma testudinarium) from central and western Japan, the JSF-endemic areas of the country (7,8). We conducted this retrospective study to determine the cause of non-JSP and non–scrub typhus disease in 2 men in western Japan who had rickettsiosis-like fever.
In 2002–2003 in Kochi Prefecture, western Japan, 2 men sought medical care for rickettsiosis-like signs and symptoms. Case-patient 1 (61 years old) sought care for fever (39.2°C), chills, and malaise 10 days after traveling to the mountains (day 0, the day of symptom onset). His physician prescribed cefdinir (300 mg/day). By day 3, signs and symptoms had not improved and an erythematous rash on his trunk had spread; the physician suspected infection with R. japonica or O. tsutsugamushi. The patient was hospitalized and intravenously administered minocycline (200 mg/day). Results (and reference values) for laboratory tests (day 3) follow: leukocytes, 5.8 × 109 cells/L (3.5–9.2 × 109 cells/L); thrombocytes, 225 × 109 cells/L (155–365 × 109 cells/L); aspartate aminotransferase, 59 U/L (<38 U/L); alanine aminotransferase, 61 U/L (<36 U/L); and C-reactive protein, 12.1 mg/dL (<0.3 mg/dL).
Case-patient 2, a 73-year-old lumberjack, sought medical care for fever (39.2°C), headache, and malaise (day 0, the day of symptom onset). On day 4, a disseminated maculopapular rash was noticed, especially on the trunk and lower limbs; JSF or scrub typhus infection was suspected. The patient was hospitalized and intravenously administered minocycline (200 mg/day). Results for laboratory tests (day 4) follow: leukocytes, 6.4 × 109 cells/L; aspartate aminotransferase, 100 U/L, alanine aminotransferase, 45 U/L; and C-reactive protein, 17.2 mg/dL.
In 2003, blood clots and serum samples from the 2 patients were transferred from Kochi Institute of Health to the University of Shizuoka, where they were stored at −20°C until a retrospective analysis could be performed. DNA was extracted from the blood clots, and nested PCR was performed, as described (3,9), to detect SFG rickettsiae 16S rDNA, O. tsutsugamushi 16S rDNA, A. phagocytophilum p44/msp2, and Ehrlichia spp. p28/omp-1 (Table 1). To avoid DNA contamination, we performed PCR, electrophoresis, and cloning were performed in separate laboratories. As a negative control, nested PCR without DNA template samples was performed for each sample. PCR detected A. phagocytophilum p44/msp2 multigenes in acute-phase blood clots from both case-patients, and SFG rickettsia 16S rDNA was amplified from a sample from case-patient 2 (Table 1).
Amplicons of p44/msp2 were subjected to TA cloning (TA Cloning Kit; Life Technologies, Grand Island, NY, USA), and randomly selected recombinant clones were sequenced and analyzed phylogenetically (Figure 1). A total of 28 p44/msp2 clones from case-patient 1 shared 27.5%–100% similarity with each other and were widely dispersed in the tree. The 40 clone sequences from case-patient 2 shared 97.5%–100% similarity with each other and grouped into a single cluster. Using Blast (http://blast.ncbi.nlm.nih.gov), we compared the sequences with those in GenBank; 27 previously identified p44/msp2 variants from human isolates and ticks collected in Japan were identified as the closest relatives to p44/msp2 cloned from the 2 patients. We included the 27 variants in the tree; however, some were widely separated from the related clones (Figure 1). For case-patient 2, the 389-bp sequence of the 16S rDNA amplicon (determined by direct sequencing) was 100% identical to that of R. japonica YH (GenBank accession no. AP011533).
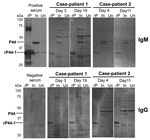
Serologic evidence of infection was demonstrated by using indirect immunofluorescence assay (IFA) and Western blot analysis as described (10,11). In IFAs, IgM and/or IgG from serum samples from the case-patients reacted with A. phagocytophilum cultured in THP-1 rather than HL60 cells, and seroconversion was stronger in convalescent-phase serum samples (Table 2). IgG titers against R. japonica were also higher in convalescent-phase samples from case-patient 2. Western blot analysis further confirmed the specific reaction to the 44-kDa outer membrane proteins (P44s) of A. phagocytophilum cultured in THP-1 cells and/or to the recombinant P44-1 (rP44-1) in serum samples (Figure 2, Table 2). However, using the same serum samples, we could not detect P44 antigens of A. phagocytophilum propagated in HL60 cells (data not shown), supporting the IFA result.
In central and western Japan, most cases of tickborne infectious and febrile disease have been reported as JSF (1,13), and R. japonica has been frequently detected in ixodid ticks in these areas. We found A. phagocytophilum infection in several species of ticks, and at least 3 species (H. formosensis, H. longicornis, and I. ovatus) seem to be associated with R. japonica and A. phagocytophilum (7,8). National surveillance during 1999–2010, showed that JSF was endemic in Kochi Prefecture during 1999–2004. More recently, JSF-endemic areas are Mie, Kagoshima, Wakayama, and Kumamoto Prefectures rather than Kochi Prefecture. Our survey demonstrating the presence of A. phagocytophilum–infected ticks in Mie and Kagoshima Prefectures (8) indicates that there is a risk for dual infection with R. japonica and A. phagocytophilum in JSF-endemic areas of Japan.
A. phagocytophilum cultured in HL60 cells is generally used as a source of antigen for serodiagnosis of human anaplasmosis. Our findings show, however, that titers of antibody against A. phagocytophilum propagated in THP-1 cells were higher than those propagated in HL60 cells. We further analyzed the transcription of p44/msp2 multigenes encoding P44 repertoires (major antigens of A. phagocytophilum) in infected HL60 and THP-1 cells by using reverse transcription PCR followed by TA cloning as described (7). The analyses showed that a transcript from the p44-60 gene and another from the p44-47 gene (75% and 25% of transcripts tested, respectively) were dominantly expressed in A. phagocytophilum propagated in THP-1 cells but not in HL60 cells; several transcript species other than p44-60 and p44-47 of p44/msp2 multigenes were expressed in A. phagocytophilum propagated in HL60 cells (data not shown). A previous proteomic study supported the variety of P44 repertoires produced by A. phagocytophilum in HL60 cells (14). The difference of p44/msp2 expression between HL60 and THP-1 cell cultures may reflect the discrepancy of antibody titers obtained by IFAs. Furthermore, in IFAs using infected THP-1 antigens, IgM titers tended to be higher than IgG titers, even in convalescent-phase serum samples. These patients probably produced IgG reactive with P44 species other than P44-60 and P44-47 that were dominantly expressed in A. phagocytophilum propagated in THP-1 cells; Western blot analysis showed that IgG in patients strongly bound to recombinant P44-1 rather than P44s (probably including P44-60 and P44-47) of A. phagocytophilum propagated in THP-1 cells. Thus, cases of human anaplasmosis could go undiagnosed if only infected HL60 cells, and not THP-1 cells, are used as antigen for serodiagnosis of rickettsiosis-like infections, as is currently done when using IFAs.
We documented 2 cases of human granulocytic anaplasmosis in Japan, 1 with and 1 without JSF coinfection. To avoid misdiagnosing cases of human anaplasmosis, we recommend that A. phagocytophilum propagated in THP-1 and in HL60 cells be used as antigens for the serodiagnosis of rickettsiosis-like infections.
Dr Ohashi is a professor in the Laboratory of Microbiology, Department of Food and Nutritional Sciences, School of Food and Nutritional Sciences, Graduate School of Integrated Pharmaceutical and Nutritional Sciences, University of Shizuoka, Japan. His primary research interests are molecular biology, ecology, and epidemiology of zoonotic parasites, especially tickborne and foodborne pathogens.
Acknowledgment
This work was supported in part by grants for Research on Emerging and Reemerging Infectious Diseases from the Association for Preventive Medicine of Japan and from the Japanese Ministry of Health, Labour and Welfare (H18-Shinkou-Ippan-014, H21-Shinkou-Ippan-006, and H24-Shinkou-Ippan-008); N.O. received a grant for the Global Center of Excellence Program from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- National Institute of Infectious Diseases, Ministry of Health, Labour and Welfare. IASR (Infectious Agents Surveillance Report). Scrub typhus and Japanese spotted fever in Japan, 2006–2011 [cited 2012 May 31]. http://idsc.nih.go.jp/iasr/31/363/tpc363.html
- Ando S, Kurosawa M, Sakata A, Fujita H, Sakai K, Sekine M, Human Rickettsia heilongjiangensis infection, Japan. Emerg Infect Dis. 2010;16:1306–8. DOIPubMedGoogle Scholar
- Ohashi N, Inayoshi M, Kitamura K, Kawamori F, Kawaguchi D, Nishimura Y, Anaplasma phagocytophilum–infected ticks, Japan. Emerg Infect Dis. 2005;11:1780–3. DOIPubMedGoogle Scholar
- Wuritu , Kawamori F, Aochi M, Masuda T, Ohashi N. Characterization of p44/msp2 multigene family of Anaplasma phagocytophilum from two different tick species, Ixodes persulcatus and Ixodes ovatus, in Japan. Jpn J Infect Dis. 2009;62:142–5.PubMedGoogle Scholar
- Wuritu , Ozawa Y, Gaowa , Kawamori F, Masuda T, Masuzawa T, Structural analysis of a p44/msp2 expression site of Anaplasma phagocytophilum in naturally infected ticks in Japan. J Med Microbiol. 2009;58:1638–44. DOIPubMedGoogle Scholar
- Kawahara M, Tajima T, Torii H, Yabutani M, Ishii J, Harasawa M, Ehrlichia chaffeensis infection of sika deer, Japan. Emerg Infect Dis. 2009;15:1991–3. DOIPubMedGoogle Scholar
- Gaowa , Wuritu , Wu D, Yoshikawa Y, Ohashi N, Kawamori F, Detection and characterization of p44/msp2 transcript variants of Anaplasma phagocytophilum from naturally infected ticks and wild deer in Japan. Jpn J Infect Dis. 2012;65:79–83.PubMedGoogle Scholar
- Gaowa , Aoch i M, Ohashi N, Wuritu , Wu D, Yoshikawa Y, Rickettsiae in Ticks, Japan, 2007–2011 [letter]. Emerg Infect Dis. 2013;19:338–40. DOIGoogle Scholar
- Inayoshi M, Naitou H, Kawamori F, Masuzawa T, Ohashi N. Characterization of Ehrlichia species from Ixodes ovatus ticks at the foot of Mt. Fuji, Japan. Microbiol Immunol. 2004;48:737–45 .PubMedGoogle Scholar
- Heo EJ, Park JH, Koo JR, Park MS, Park MY, Dumler JS, Serologic and molecular detection of Ehrlichia chaffeensis and Anaplasma phagocytophila (human granulocytic ehrlichiosis agent) in Korean patients. J Clin Microbiol. 2002;40:3082–5. DOIPubMedGoogle Scholar
- Zhi N, Ohashi N, Rikihisa Y, Horowitz HW, Wormser GP, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–73 .PubMedGoogle Scholar
- Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–9 .PubMedGoogle Scholar
- Mahara F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis. 1997;3:105–11. DOIPubMedGoogle Scholar
- Lin M, Kikuchi T, Brewer HM, Norbeck AD, Rikihisa Y. Global proteomic analysis of two tick-borne emerging zoonotic agents: Anaplasma phagocytophilum and Ehrlichia chaffeensis. Front Microbiol. 2011;2:24. Epub 2011 Feb 17. DOIGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this article.
2Current affiliation: Ehime Prefectural Central Hospital, Matsuyama, Ehime, Japan.
3Current affiliation: Kochi Medical School, Nankoku, Kochi, Japan.
Table of Contents – Volume 19, Number 2—February 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Norio Ohashi, Laboratory of Microbiology, Department of Food and Nutritional Sciences, School of Food and Nutritional Sciences, Graduate School of Integrated Pharmaceutical and Nutritional Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526, Japan
Top