Volume 20, Number 5—May 2014
Research
Streptococcus mitis Strains Causing Severe Clinical Disease in Cancer Patients
Cite This Article
Citation for Media
Abstract
The genetically diverse viridans group streptococci (VGS) are increasingly recognized as the cause of a variety of human diseases. We used a recently developed multilocus sequence analysis scheme to define the species of 118 unique VGS strains causing bacteremia in patients with cancer; Streptococcus mitis (68 patients) and S. oralis (22 patients) were the most frequently identified strains. Compared with patients infected with non–S. mitis strains, patients infected with S. mitis strains were more likely to have moderate or severe clinical disease (e.g., VGS shock syndrome). Combined with the sequence data, whole-genome analyses showed that S. mitis strains may more precisely be considered as >2 species. Furthermore, we found that multiple S. mitis strains induced disease in neutropenic mice in a dose-dependent fashion. Our data define the prominent clinical effect of the group of organisms currently classified as S. mitis and lay the groundwork for increased understanding of this understudied pathogen.
Viridans group streptococci (VGS), a genetically heterogeneous group of bacteria, are the predominant bacteria in the human oropharynx (1). VGS cause a wide range of infections in humans, including bacteremia in patients with neutropenia, infective endocarditis, and orbital cellulitis (2–5). However, despite the substantial clinical effect of VGS, the epidemiology and pathogenesis of these bacteria are minimally understood (6).
A major impediment to the study of VGS has been the inability to consistently and accurately assign VGS strains to specific species, which has resulted in numerous changes in species designation and classification schemes over time (7). From a clinical microbiology laboratory standpoint, automated systems have considerable limitations in VGS species identification (8,9). The problematic nature of VGS species assignment also extends to16S rRNA sequencing, the most widely used genetic tool for species identification in clinical and research settings (9,10).
Outcomes for patients with VGS bacteremia are highly variable: some patients have minimal symptoms, and others have a severe infection characterized by hypotension and acute respiratory distress syndrome (11). The severe infections have been termed VGS shock syndrome (12). Numerous studies have examined the species distribution of VGS that cause bacteremia (9,13–16). However, these studies have found inconsistent results between a particular VGS species and disease occurrence or clinical severity of infection (9,13,14,16,17). Moreover, the recently recognized limitations of previously used techniques of VGS species identification and the low number of clinical cases analyzed preclude definitive conclusions regarding the relationship between VGS species type and clinical disease (8,9,18). Thus, we sought to combine the species identification of a large number of VGS bloodstream isolates, which we typed by using a recently developed multilocus sequence analysis (MLSA) technique (19), with patient-specific clinical data to determine relationships between VGS species and clinical endpoints.
Materials and Methods
Study Cohort and Data Abstraction
The study cohort comprised patients at MD Anderson Cancer (MDACC) who had VGS isolated from their blood between July 1, 2011, and December 1, 2012. MDAAC is a 600-bed referral cancer hospital in Houston, Texas, USA. We used a standardized data collection form to abstract clinical data from the comprehensive electronic medical records of patients with blood culture results positive for VGS. Antimicrobial drug resistance was determined in accordance with guidelines of the Clinical and Laboratory Standards Institute (www.clsi.org/standards/). VGS are known to contaminate blood cultures and to cause clinically minor, transient bacteremia, and differentiating between contamination and infection is problematic (20). Thus, for the purpose of this study, we considered that patients without signs or symptoms of infection had clinically minor bacteremia, even though they may represent cases of blood culture contamination.
Severity of infection, as measured by the Pitt bacteremia score, was determined as described (21). Pitt bacteremia scores were not determined for patients with polymicrobial bacteremia. VGS shock syndrome was defined by using the accepted definition for septic shock (i.e., hypotension refractory to fluid replacement in the setting of an infection) (22). A focus of the bloodstream infection was defined as isolation of a VGS species from a nonsterile site (e.g., liver abscess) at the same time that VGS were isolated from the blood, with the exception of infective endocarditis, which was defined according the modified Duke criteria (23,24). Neutropenia was defined as an absolute neutrophil count of <500 cells/μL.
Some patients had signs and symptoms of a lower respiratory infection and x-ray findings compatible with a pneumonic process that could not be explained (i.e., no known respiratory pathogens were isolated and no other alternative explanation, e.g., congestive heart failure, was found). Such patients were defined as having unexplained pulmonary infiltrates. Because VGS are considered normal flora, isolation of these organisms from a respiratory specimen would not have been considered clinically meaningful by the clinical microbiology laboratory and thus would not have been reported. The study protocol was approved by the MDCC institutional review board.
VGS Species Type Determination and Whole-Genome Sequencing
Bacterial isolates were identified as VGS on the basis of the following: presence of α-hemolysis, gram-positive reaction, coccus morphology arranged in chains, negative catalase test results, and exclusions of pneumococcus and enterococci by routine biochemical tests (i.e., optochin, bile solubility, and pyrrolidonyl arylamidase tests) (25). VGS species was determined as described (19). In brief, concatenated sequences of 7 housekeeping genes were used for phylogenetic tree construction in MEGA5 (megasoftware.net/); strains were assigned to VGS species on the basis of their distance from species type strains (19). For whole-genome sequencing of 9 Streptococcus mitis strains and 1 S. oralis strain, we fragmented 3 μg of genomic DNA to 350 bp (mean fragment size) and prepared barcoded sequencing libraries. The 03/10libraries were sequenced on the HiSeq 2000 sequencing System (Illumina, San Diego, CA, USA) by using 76-bp, paired-end sequencing. The raw reads in FASTQ format were aligned to the S. mitis B6 (GenBank accession no. NC_013853.1) and S. pneumoniae TIGR4 (GenBank accession no. NC_003028.3) genomes by using Mosaik (26). There was an average of 250× coverage per base, indicating extremely high confidence for base calls. Contigs were generated by feeding the raw genome sequence data into the A5 pipeline (27). Gene annotations were obtained by uploading contigs to the Rapid Annotation using the Subsystem Technology server at the National Microbial Pathogen Data Resource website (28). (Individual gene sequencing data have been deposited into GenBank. Short-read sequencing data have been deposited to the Short Read Archive (www.ncbi.nlm.nih.gov/Traces/sra/sra.cgi?view=announcement) under accession no. PRJNA240080.)
Mouse Infection Studies
Experiments in mice were performed according to a protocol approved by the MDACC Institutional Animal Care and Use Committee. To induce neutropenia, we injected 5-week-old female Balb/C mice intraperitoneally with 100 mg/kg of cyclophosphamide (Sigma, St. Louis, MO, USA) on days −4 and −1 before bacterial injection. On day 0, mice (10 per bacterial dose) were injected with 100 μL of phosphate-buffered saline (PBS) containing 10-fold increments of bacteria ranging from 103 to 107 CFUs. As a control, 10 mice were injected with PBS alone. Mice were monitored over 7 days for near-death status. The dose at which 50% of the mice nearly died (hereafter referred to as LD50) was calculated by using the probit method. Neutropenia was confirmed in select mice on postinfection days 1, 3, and 6.
Statistical Analysis
Differences between categorical variables were assessed by using the χ2 test; Fisher exact test was used when at least 1 category had <5 occurrences. The relationship between VGS species and Pitt bacteremia scores was analyzed by using the Mann-Whitney U test. The Bonferroni method was employed to account for multiple comparisons when appropriate. All tests of significance were 2-sided, and statistical significance was defined at p<0.05. SPSS Statistics version 19 (IBM, Armonk, NY, USA ) was used for statistical analysis.
Results
Study Cohort
A total of 118 consecutive patients with VGS-positive blood cultures were included in the study cohort; ≈80% of the patients had neutropenia and hematologic malignancies (Table 1). Most patients had bacteremia without a defined focus, but several other clinical scenarios were observed, including skin/soft tissue infections, gastrointestinal infections, and infective endocarditis. Most patients had clinically mild infections (Pitt bacteremia score of 0 or 1), but 25% of patients had moderate to severe infections (Pitt bacteremia scores of >2), including 12 patients who had VGS shock syndrome.
VGS Species and Bacteremia
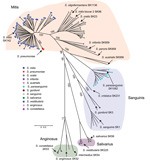
To gain insight into the species of VGS causing bacteremia in the study cohort, we performed MLSA of 7 housekeeping genes, as described (19). Strains were assigned to species by comparing their position on the phylogenetic tree with those of established type strains (19). The 118 strains could be confidently assigned to 11 distinct species (Figure 1; Technical Appendix 1). The most commonly observed species were S. mitis (68 strains), S. oralis (22 strains), and S. parasanguinis (12 strains). For classification purposes, various VGS species are often placed into distinct groups; the association between VGS strains causing bacteremia and group assignment is shown in Figure 1, using the scheme from Sinner et al. (29). In total, 80% of strains were from the Mitis group, and the remaining strains were from the Sanguinis group (14%), Anginosus group (3%), and Salivarius group (3%).
VGS Species and Clinical Syndromes
Because of the diverse genetic nature of the various VGS species, we next tested the hypothesis that distinct VGS species cause different clinical syndromes. Given the number of strains for each species, we analyzed the Mitis group species (i.e., S. mitis and S. oralis) individually and analyzed species comprising the Sanguinis, Anginosus, and Salivarius groups by group (Table 2). Compared with strains of other VGS species, S. mitis strains were significantly more likely to cause primary bacteremia (p<0.01) and less likely to cause polymicrobial bacteremia (p = 0.01) and clinically minor bacteremia (p<0.01). S. oralis strains were more likely to cause polymicrobial infection (p = 0.02), Sanguinis group strains were more likely to cause clinically minor bacteremia (p<0.01), and Anginosus group strains were significantly associated with bacteremia with a gastrointestinal focus (p<0.01). When we only considered patients with neutropenia or cases of monomicrobial bacteremia, we observed the same statistically significant species–clinical disease relationships (data not shown).
VGS Species and Disease Severity
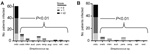
We next sought to determine if there was a relationship between VGS species and disease severity (as determined by Pitt bacteremia score). Organ dysfunction, such as hypotension, begins to occur at Pitt bacteremia scores of >2 (21). The distribution of Pitt bacteremia score by infecting species is shown in Figure 2, panel A. Patients infected with S. mitis were significantly more likely to have a higher Pitt bacteremia score (p<0.01). One possible explanation for this observation is that S. mitis strains mainly caused infections in patients with neutropenia who, compared with patients without neutropenia, might be more likely to have serious infections. Thus, we repeated the analysis, including only patients with neutropenia. Again, the Pitt bacteremia scores were significantly higher for patients infected with S. mitis (p<0.01; Figure 2, panel B). Of the 12 cases of VGS shock syndrome, 11 were caused by S. mitis strains and 1 was caused by an S. constellatus strain (Anginosus group).
Identification of S. mitis Strain Clusters
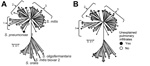
Most cases of bacteremia and severe disease occurred in patients infected with S. mitis; thus, we focused on S. mitis strains and strains from the closely related S. oralis species. In contrast to what we observed for the S. oralis strains, several distinct groupings could be visualized within the S. mitis strains, which we arbitrarily labeled as clusters 1, 2, and 3 (Figure 3, panel A). S. mitis cluster 1 comprised 10 strains, including 2 that were genetically identical by MLSA, and cluster 2 comprised 22 strains, including 6 that were genetically identical. Cluster 3 comprised 29 strains and may contain additional strain groupings, but further phylogenetic delineation of this cluster could not be done with sufficient confidence. We did not observe substantial differences, in terms of distinct disease types or severity of infection, between patients from whom the S. mitis cluster strains were derived (Technical Appendix 2). However, there was a predominance of unexplained pulmonary infiltrate cases among patients infected with cluster 2 strains (p<0.01 for cluster 2 strains vs. noncluster 2 strains) (Figure 3, panel B). This variable was investigated because, given the close genetic relationship between S. mitis and S. pneumoniae, we hypothesize that S. mitis strains may cause pneumonia in severely immunocompromised persons (Figure 1).
Whole-Genome Analysis and MLSA Grouping of Strains
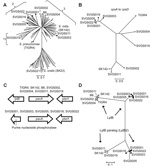
To determine whether the MLSA data accurately represented the entire genetic content of the Mitis group strains, we performed whole-genome sequencing of 9 S. mitis and 1 S. oralis isolates (Figure 4, panel A). For the 9 S. mitis strains, the reads mapped to ≈70% coverage of the only completely finished S. mitis genome (S. mitis strain B6 [30]), Technical Appendix 2, Table 1). This considerable level of intraspecies genetic diversity for S. mitis strains has been observed previously in sequencing and DNA:DNA hybridization studies and meant that we could not use whole-genome analysis of single-nucleotide polymorphisms to determine strain relatedness (30,31). Thus, we next sought to identify regions of genetic similarity among the strains that could be analyzed for interstrain comparisons.
All 9 S. mitis strains contained operons encoding a putative polysaccharide capsule. The first 4 genes of the operon, corresponding to cpsA–cpsD in S. pneumoniae, were relatively well conserved among the 9 strains. Concatenated alignment of cpsA–cpsD showed a close relationship for the 4 cluster 2 strains and strain Shelburne VGS (SVGS) 003, whereas the cpsA–cpsD genes from the remaining 4 strains were more closely related to the S. pneumoniae strain TIGR4 (SVGS004 and SVGS019) or to the S. mitis type strain SK142 (SVGS002 and SVGS011) (Figure 4, panel B).
In addition to the capsule operons, multiple other comparisons arising from our whole-genome analysis confirmed the idea that the 4 cluster 2 strains and strain SVGS003 were closely related. All of the S. mitis strains contained the gene encoding the fibrinogen-binding protein, PavA (pneumococcal adherence and virulence protein A). However, there was a different gene 5′ to the pavA gene in SVGS003 and the 4 cluster 2 strains than in the other 4 S. mitis strains (Figure 4, panel C). In a similar manner, the LytB protein in cluster 2 strains and SVGS003 grouped separately from the LytB protein in the other strains, and a LytB paralog in strain SVGS003 and the 4 cluster 2 strains was distinct from other forms of the LytB protein and from LytB paralogs encoded by SVGS011 and SVGS019.
LytB is part of a group of choline-binding proteins that are involved in cell-wall turnover, some of which have been shown to be important for virulence in S. pneumoniae (32). When the presence or absence of choline-binding proteins was determined for the various strains, substantial strain-to-strain heterogeneity was observed (Technical Appendix 2 Table 2). The only repeating pattern of gene content was 1 that occurred for 3 different choline-binding protein–encoding genes: cbpE, cbpI, and lytC. These 3 genes, which are present in diverse chromosomal locations, were absent in the 4 cluster 2 strains, SVGS003, and the S. oralis strain SVGS021 but present in the other noncluster 2 S. mitis strains. Thus, the whole-genome data support MLSA data, assigning 4 of the fully sequenced strain to S. mitis cluster 2; strain SVGS003, a group 3 strain by MLSA, appears to have genetic characteristics of the cluster 2 strains by whole-genome analysis.
Mouse Model for Testing VGS Virulence
Given the apparent differences in genetic content among S. mitis strains, we sought to develop an animal model for testing VGS virulence that would approximate the disease observed in cancer patients with neutropenia. No neutropenia model of VGS infection exists, so we used serial 10-fold CFU dilutions of 5 S. mitis strains to determine the LD50 of organisms for the endpoint of being near death. The S. mitis challenge strains included isolates from the major S. mitis clusters (Figure 5; Table 3), and we also injected PBS as a control. None of the mice injected with PBS became ill, indicating that neither the neutropenia nor the injection itself caused major disease (Figure 5, panel B). All of the S. mitis strains could cause near-death status, and a dose–response relationship was observed for all strains (see example in Figure 5, panel B), but the LD50 varied by 100-fold among the strains (Table 3). Strain SVGS016, which caused the most severe clinical disease (i.e., it was isolated from a patient with the highest Pitt bacteremia score) also was the most virulent in the mouse model. Thus, we suggest that S. mitis strains cause disease in mice with neutropenia and that there is differential virulence in this mouse model among genetically diverse S. mitis strains.
Since first being identified as causative agents of infections in cancer patients with neutropenia ≈35 years ago (33), VGS have come to be appreciated as major bacterial pathogens in patients with malignancy (2,12,14,34,35). The emergence of VGS as common infectious agents has coincided with the increasing use of prophylactic antimicrobial drugs, especially fluoroquinolones, for patients with neutropenia (36). However, despite the clear clinical consequences of VGS infections, there is minimal understanding of their pathophysiology.
A critical first step in the study of VGS is to define the clinical syndromes caused by various VGS species. This goal has long been hampered by difficulties in using phenotypic methods or single-gene sequencing approaches to assign VGS strains to particular species (18). Through the use of a recently developed MLSA approach (19), we showed that there is a relationship between VGS species, as defined genetically, and disease manifestations in patients with cancer (Table 2). An unexpected finding was the relationship between Sanguinis group strains and clinically minor bacteremia; Sanguinis group species are the leading VGS cause of infective endocarditis and have been reported to cause virulent infections in patients with neutropenia (17,37). One possible explanation for this finding is that Sanguinis group VGS are often causative agents of transient bacteremia and that transient bacteremia occasionally results in infective endocarditis. Platelets are thought to be critical to the pathogenesis of VGS infective endocarditis. Thus, because of low platelet counts, persons with cancer, especially those with hematologic malignancy, may be relatively resistant to the development of infective endocarditis after transient VGS bacteremia (38).
Another key relationship that we observed was that of S. mitis and primary bacteremia during periods of neutropenia. Our data support and extend the findings of other smaller studies using genetic techniques that found a similar predominance of S. mitis strains in patients with neutropenia (9,15,39). The reason that S. mitis strains are the leading cause of VGS bacteremia in patients with neutropenia is not known. One could postulate that S. mitis is simply the dominant commensal VGS species and thus is the most likely species to translocate across epithelial barriers when patients become neutropenic. Indeed, a recent microbiome study showed that S. mitis is the predominant VGS species isolated from buccal mucosa samples from healthy persons (1). However, in our study, S. mitis not only caused the majority of neutropenic infections but also caused a disproportionate percentage of serious infections (Figure 2). Thus, at least for patients with neutropenia, S. mitis is more likely than other VGS to enter into the bloodstream and to cause serious infections once there. Moreover, compared with other VGS species, S. mitis rarely caused clinically minor bacteremia or polymicrobial infection, suggesting that S. mitis strains have inherently virulent properties compared with other VGS. The data from our multistrain, whole-genome sequencing and the development of an animal model of neutropenia and S. mitis infection should provide a key platform for elucidating S. mitis virulence.
The deep branching pattern produced by MLSA of our S. mitis strains isolated from human blood has been observed in other investigations (19,30) and suggests that the organisms currently grouped as S. mitis may more precisely be considered as >2 species. The application of whole-genome sequencing to large numbers of S. mitis strains will be necessary to fully resolve S. mitis strain clusters, as shown by the somewhat discordant results of our MLSA and whole-genome analysis. In addition, we were intrigued by the association of cluster 2 S. mitis strains and unexplained pneumonia (Figure 3, panel B). Given the close genetic relationship between S. mitis and S. pneumoniae, it might be expected that some S. mitis strains could cause pneumonia, especially in severely immunocompromised patients. Whether particular subspecies of S. mitis can cause pneumonia is an active area of investigation in our laboratory, and if it does, that could help explain the stubbornly low number of pathogens that can be identified for patients with pneumonic syndromes (40).
This large series of invasive VGS strains includes detailed molecular and clinical information. By combining these 2 sets of data, we have definitively established the critical role of S. mitis strains in invasive VGS infection in patients with cancer and have laid the groundwork for future insights into how these organisms cause serious disease in vulnerable hosts.
Dr Shelburne is an associate professor and physician–scientist at MD Anderson Cancer Center. His research interest is in the influences of bacterial genetics on the clinical signs and symptoms of infectious diseases in humans.
Acknowledgments
We thank the clinical microbiology laboratory staff at MD Anderson Cancer Center for their efforts in identifying and saving VGS isolates and Nathaniel Albert and Dimitrios Kontoyiannis for assistance with the neutropenic mouse model.
This study was supported by an internal grant (to S.A.S.) from MD Anderson Cancer Center. H.Y. is supported by the H. A. and Mary K. Chapman Foundation.
References
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14 . DOIPubMedGoogle Scholar
- Tunkel AR, Sepkowitz KA. Infections caused by viridans streptococci in patients with neutropenia. Clin Infect Dis. 2002;34:1524–9 . DOIPubMedGoogle Scholar
- Dix D, Cellot S, Price V, Gillmeister B, Ethier MC, Johnston DL, Association between corticosteroids and infection, sepsis, and infectious death in pediatric acute myeloid leukemia (AML): results from the Canadian Infections in AML Research Group. Clin Infect Dis. 2012;55:1608–14. DOIPubMedGoogle Scholar
- Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis–Prospective Cohort Study. Arch Intern Med. 2009;169:463–73 . DOIPubMedGoogle Scholar
- Seltz LB, Smith J, Durairaj VD, Enzenauer R, Todd J. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566–72. DOIPubMedGoogle Scholar
- Seo HS, Xiong YQ, Mitchell J, Seepersaud R, Bayer AS, Sullam PM. Bacteriophage lysin mediates the binding of Streptococcus mitis to human platelets through interaction with fibrinogen. PLoS Pathog. 2010;6:e1001047. DOIPubMedGoogle Scholar
- Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–30 . DOIPubMedGoogle Scholar
- Teles C, Smith A, Ramage G, Lang S. Identification of clinically relevant viridans group streptococci by phenotypic and genotypic analysis. Eur J Clin Microbiol Infect Dis. 2011;30:243–50. DOIPubMedGoogle Scholar
- Hoshino T, Fujiwara T, Kilian M. Use of phylogenetic and phenotypic analyses to identify nonhemolytic streptococci isolated from bacteremic patients. J Clin Microbiol. 2005;43:6073–85. DOIPubMedGoogle Scholar
- Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–8 . DOIPubMedGoogle Scholar
- Bochud PY, Eggiman P, Calandra T, Van Melle G, Saghafi L, Francioli P. Bacteremia due to viridans streptococcus in neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis. 1994;18:25–31 . DOIPubMedGoogle Scholar
- Gassas A, Grant R, Richardson S, Dupuis LL, Doyle J, Allen U, Predictors of viridans streptococcal shock syndrome in bacteremic children with cancer and stem-cell transplant recipients. J Clin Oncol. 2004;22:1222–7. DOIPubMedGoogle Scholar
- Wisplinghoff H, Reinert RR, Cornely O, Seifert H. Molecular relationships and antimicrobial susceptibilities of viridans group streptococci isolated from blood of neutropenic cancer patients. J Clin Microbiol. 1999;37:1876–80 .PubMedGoogle Scholar
- Elting LS, Bodey GP, Keefe BH. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis. 1992;14:1201–7. DOIPubMedGoogle Scholar
- Kitten T, Munro CL, Zollar NQ, Lee SP, Patel RD. Oral streptococcal bacteremia in hospitalized patients: taxonomic identification and clinical characterization. J Clin Microbiol. 2012;50:1039–42. DOIPubMedGoogle Scholar
- Bilgrami S, Feingold JM, Dorsky D, Edwards RL, Clive J, Tutschka PJ. Streptococcus viridans bacteremia following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1998;21:591–5. DOIPubMedGoogle Scholar
- Husain E, Whitehead S, Castell A, Thomas EE, Speert DP. Viridans streptococci bacteremia in children with malignancy: relevance of species identification and penicillin susceptibility. Pediatr Infect Dis J. 2005;24:563–6. DOIPubMedGoogle Scholar
- Ikryannikova LN, Lapin KN, Malakhova MV, Filimonova AV, Ilina EN, Dubovickaya VA, Misidentification of alpha-hemolytic streptococci by routine tests in clinical practice. Infect Genet Evol. 2011;11:1709–15 . DOIPubMedGoogle Scholar
- Bishop CJ, Aanensen DM, Jordan GE, Kilian M, Hanage WP, Spratt BG. Assigning strains to bacterial species via the Internet. BMC Biol. 2009;7:3 . DOIPubMedGoogle Scholar
- Swenson FJ, Rubin SJ. Clinical significance of viridans streptococci isolated from blood cultures. J Clin Microbiol. 1982;15:725–7 .PubMedGoogle Scholar
- Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum β-lactamase production in nosocomial Infections. Ann Intern Med. 2004;140:26–32 . DOIPubMedGoogle Scholar
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest. 2009;136(Suppl):e28 .PubMedGoogle Scholar
- Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008;299:2056–65. DOIPubMedGoogle Scholar
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. DOIPubMedGoogle Scholar
- Han XY, Kamana M, Rolston KV. Viridans streptococci isolated by culture from blood of cancer patients: clinical and microbiologic analysis of 50 cases. J Clin Microbiol. 2006;44:160–5. DOIPubMedGoogle Scholar
- Hillier LW, Marth GT, Quinlan AR, Dooling D, Fewell G, Barnett D, Whole-genome sequencing and variant discovery in C. elegans. Nat Methods. 2008;5:183–8. DOIPubMedGoogle Scholar
- Tritt A, Eisen JA, Facciotti MT, Darling AE. An integrated pipeline for de novo assembly of microbial genomes. PLoS ONE. 2012;7:e42304. DOIPubMedGoogle Scholar
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. DOIPubMedGoogle Scholar
- Sinner SW, Tunkel AR. Viridans group streptococci, groups C and G streptococci, and Gemella morbilliform. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia: Elsevier; 2011. p. 2434–50.
- Denapaite D, Bruckner R, Nuhn M, Reichmann P, Henrich B, Maurer P, The genome of Streptococcus mitis B6—what is a commensal? PLoS ONE. 2010;5:e9426. DOIPubMedGoogle Scholar
- Kilian M, Poulsen K, Blomqvist T, Havarstein LS, Bek-Thomsen M, Tettelin H, Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS ONE. 2008;3:e2683. DOIPubMedGoogle Scholar
- Pérez-Dorado I, Galan-Bartual S, Hermoso JA. Pneumococcal surface proteins: when the whole is greater than the sum of its parts. Mol Oral Microbiol. 2012;27:221–45.
- Pizzo PA, Ladisch S, Witebsky FG. Alpha-hemolytic streptococci: clinical significance in the cancer patient. Med Pediatr Oncol. 1978;4:367–70. DOIPubMedGoogle Scholar
- Burden AD, Oppenheim BA, Crowther D, Howell A, Morgenstern GR, Scarffe JH, Viridans streptococcal bacteraemia in patients with haematological and solid malignancies. Eur J Cancer. 1991;27:409–11. DOIPubMedGoogle Scholar
- Villablanca JG, Steiner M, Kersey J, Ramsay NK, Ferrieri P, Haake R, The clinical spectrum of infections with viridans streptococci in bone marrow transplant patients. Bone Marrow Transplant. 1990;5:387–93 .PubMedGoogle Scholar
- Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39(Suppl 1):S25–31 . DOIPubMedGoogle Scholar
- Roberts RB, Krieger AG, Schiller NL, Gross KC. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979;1:955–66 . DOIPubMedGoogle Scholar
- Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis. 2012;205:1066–75. DOIPubMedGoogle Scholar
- Westling K, Julander I, Ljungman P, Vondracek M, Wretlind B, Jalal S. Identification of species of viridans group streptococci in clinical blood culture isolates by sequence analysis of the RNase P RNA gene, rnpB. J Infect. 2008;56:204–10. DOIPubMedGoogle Scholar
- Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect. 2013;67:11–8 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 20, Number 5—May 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Samuel A. Shelburne, Department of Infectious Diseases, Department of Genomic Medicine, MD Anderson Cancer Center, Unit 1460, 1515 Holcombe Blvd, Houston, TX, USASamuel A. Shelburne, Department of Infectious Diseases, Department of Genomic Medicine, MD Anderson Cancer Center, Unit 1460, 1515 Holcombe Blvd, Houston, TX 77030-4095, USA
Top