Volume 22, Number 5—May 2016
Research
Expansion of Shiga Toxin–Producing Escherichia coli by Use of Bovine Antibiotic Growth Promoters
Abstract
Antibiotics are routinely used in food-producing animals to promote growth and prevent infectious diseases. We investigated the effects of bovine antibiotic growth promoters (bAGPs) on the propagation and spread of Shiga toxin (Stx)–encoding phages in Escherichia coli. Co-culture of E. coli O157:H7 and other E. coli isolated from cattle in the presence of sublethal concentrations of bAGPs significantly increased the emergence of non-O157, Stx-producing E. coli by triggering the SOS response system in E. coli O157:H7. The most substantial mediation of Stx phage transmission was induced by oxytetracyline and chlortetracycline, which are commonly used in agriculture. bAGPs may therefore contribute to the expansion of pathogenic Stx-producing E. coli.
Antimicrobial agents are commonly used subtherapeutically as feed supplements to promote growth and to prevent infection in livestock. Despite growing public health concerns about resistance associated with agricultural use of antibiotics, their use in livestock production is anticipated to increase (1). Each year in the United States, 1,675 tons of nontherapeutic antibiotics are used in beef cattle, particularly in feedlots, which are intensive cattle-raising systems (2). According to a report from the US Department of Agriculture, ionophores, tylosin, chlortetracycline, and oxytetracycline are frequently given to feedlot cattle (3). Ionophores, such as monensin, are included in feed mainly to increase weight gain and to prevent bovine coccidiosis (3,4). Tylosin is used to prevent diseases (e.g., hepatic abscessation) and to promote growth in cattle (3,5), whereas chlortetracycline and oxytetracycline are used as feed supplements mainly to prevent bovine pneumonia and bacterial enteritis (4). Although antibiotics are usually added to feed, water, or both at subtherapeutic levels, at some feedlots, chlortetracycline and oxytetracycline are used at therapeutic levels to prevent infection, particularly when calves are first introduced into feedlots (6).
Shiga toxin–producing Escherichia coli (STEC), such as E. coli O157:H7, is the leading cause of hemorrhagic colitis and hemolytic uremic syndrome (7). Shiga toxin (Stx) is reportedly produced by ≈250 different O serotypes of E. coli; non-O157 STEC infection is becoming increasingly prevalent, accounting for up to 20%–50% of STEC infections in the United States (8). In particular, 6 serogroups (O26, O45, O103, O111, O121, and O145) are responsible for 83% of all non-O157 infections in the United States (9). The stx genes are encoded by lambdoid bacteriophages (10). Because secretion systems for Stx are lacking, the release of Stx is mediated through bacterial cell lysis by Stx phages in response to the induction of the SOS response (a cellular response to DNA damage) (11). Antimicrobial agents, particularly those that interfere with DNA synthesis (e.g., quinolones and trimethoprim), enhance the propagation of Stx phages and consequently increase Stx production (12). For this reason, antimicrobial drug treatment is not recommended for patients with enterohemorrhagic E. coli infection (13). In contrast, antibiotics are widely used as feed supplements in cattle, which are the primary natural reservoir for O157 and non-O157 STEC strains (14,15). E. coli is highly prevalent in cattle feces at levels ranging from 107 to 109 CFU/g (16), and E. coli O157 primarily colonizes the terminal rectum in cattle and is found in cattle feces at 103–105 CFU/g (17). Unlike humans, cattle are not susceptible to STEC infection because they lack Stx receptors (18); thus, antibiotics do not generate clinical problems in cattle. However, bovine antibiotic growth promoters (bAGPs) may induce the propagation of Stx phages and consequently facilitate the horizontal transfer of stx genes in E. coli. In this study, we investigated whether bAGPs can affect the propagation of Stx phages and contribute to the diversification of Stx-producing E. coli.
E. coli Strains, Plasmids, and Culture Conditions
We routinely maintained E. coli O157:H7 EDL933 and all E. coli isolates from cattle in Luria Bertani (LB) medium. The plasmid u66recA, a transcriptional fusion of recA::egfp, is described elsewhere (19). Detoxified EDL933 strains (Δstx2::Km and Pstx2::gfp in which stx2 is replaced with a kanamycin resistance cassette and gfp, respectively) were constructed according to a method described by Datsenko and Wanner (20). The stx2 promoter region was PCR amplified from E. coli EDL933 with Pro_Stx2-F and Pro_Stx2-R primers (Table 1). The resulting 377-bp PCR product was purified, digested with XbaI, and ligated to an XbaI site located immediately upstream of the promoterless gfp gene in pFPV25.1 (23). Pstx2::gfp was prepared by PCR with GFP_BGL_F and GFP_BGL_R (Table 1). The PCR product was cloned to a BglII site upstream of the flippase recognition target (FRT) in pKD13 (20). The FRT-flanked Pstx2::gfp was amplified with PCR from pKD13 by use of pKD13-F and pKD13-R primers, and the amplicon was introduced to EDL933 harboring pKD46 by electroporation. The transcriptional Pstx2::gfp fusion was constructed by replacing the stx2 gene with gfp in EDL933. The pKD46 plasmid was cured from the mutant by culturing at 37°C. A Δstx2::Km mutant of E. coli O157:H7 EDL933 was constructed by replacing stx2 with a FRT-flanked kanamycin resistance cassette that had been PCR amplified from pKD13. The FRT-Km-FRT amplicon was introduced into EDL933 harboring pKD46 by electroporation (20). The allelic exchange was confirmed by PCR with the primer sets of Stx2-F and Stx2-R, Kt and K1. We added tetracycline (50 μg/mL), ampicillin (100 μg/mL), and kanamycin (50 μg/mL) to culture media when necessary.
Antibiotics
Monensin, tylosin, chlortetracycline, oxytetracycline, neomycin, and sulfamethazine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ciprofloxacin was purchased from Enzo Life Sciences Inc. (Farmingdale, NY, USA).
Measurement of Pstx2::gfp Expression
Cultures carrying the Pstx2::gfp promoter fusion were collected in the exponential phase by centrifugation (5 min at 6000 × g), and bacterial cells were resuspended in fresh LB medium to ≈4.0 × 107 CFU/mL. After growing in LB broth supplemented with various concentrations of bAGPs and ciprofloxacin for 3 h, 200 µL samples were transferred to each well in a 96-well microplate and green fluorescent protein wavelength was measured with a fluorometer (FLUOstar Omega, BMG Labtech, Ortenberg, Germany). Fluorescence was monitored at excitation and emission wavelengths of 520 nm and 480 nm, respectively. Fluorescence intensities are reported in the instrument’s relative fluorescence units. The expression level of recA was measured in the same way with u66recA. The experiments were performed with triplicate samples and repeated at least 3 times.
Stx Phage Induction
Stx phages were induced by monensin, tylosin, chlortetracycline, oxytetracycline, and ciprofloxacin. Because ciprofloxacin is a well-known Stx phage inducer (12), we used ciprofloxacin as a control. Lysogenic strains were grown in LB broth overnight at 37°C with shaking and diluted to an optical density at 600 nm of 0.1 in NZCYM broth (Amresco, Solon, OH, USA). The cultures were incubated at 37°C with shaking (200 rpm) for 18 h in the presence (0.01, 0.1, and 1 μg/mL) and absence (control) of the 5 antibiotics. After centrifugation at 5,000 × g, the supernatant was sterilized with a 0.22-μm filter and used immediately. We then added 10-fold serial dilutions of phage lysates to 1 mL of E. coli C600 at the stationary phase. Then, 3 mL of top agar supplemented with 5 mmol/L calcium chloride was added to this culture and the mixture was poured on an LB agar plate. The plates were incubated at 37°C overnight, and PFU were counted the next day.
Stx Phage Transfer Assay
We investigated the transfer of Stx phages in the presence of bAGPs at different concentrations. Briefly, 5 mL of E. coli EDL933 Δstx2::Km (donor) and 6 stx2-negative bovine E. coli strains (recipients) with ampicillin or tetracycline resistance were grown in LB broth at 37°C overnight. Overnight cultures were diluted 100-fold in fresh NZCYM broth and cultured until the early exponential phase for 3 h. The donor strain (≈104 CFU/mL) was mixed with recipient strains (≈107 CFU/mL) in the presence of different concentrations of bAGPs. The mixed cultures were incubated at 37°C overnight without shaking. After incubation, 100 µL of culture was spread onto sorbitol-MacConkey agar plates supplemented with kanamycin and ampicillin or tetracycline and incubated at 37°C overnight. We calculated the transduction frequencies by dividing the number of transductants by the number of recipients.
Characterization of Transductants
Pink colonies growing on sorbitol-MacConkey agar supplemented with either kanamycin and tetracycline or kanamycin and ampicillin were regarded as presumptive transductants (i.e., recipients of the stx2-encoding phage 933W). The presumptive transductants of the stx2-encoding phage 933W were verified by performing multiplex PCR. PCRs were performed with specific primer pairs: Stx2-F and Stx2-R for the stx2 gene in 933W, eaeA-F and eaeA-R for eaeA encoding intimin (21), Ec1-uspA and Ec2-uspA for the uspA gene encoding the universal stress protein in E. coli (22), and O157F and O157R for a region in rfbE (O-antigen-encoding) for the O157 serotype (21). Serologic tests were performed with O157 and O26 antiserum (Korea National Institute of Health, Osong, South Korea).
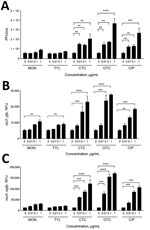
Enhanced Propagation of Stx Phages in E. coli O157:H7 by bAGPs
To investigate the effect of subtherapeutic concentrations of bAGPs on the propagation of Stx phages, we first measured the level of Stx phage propagation after exposure of E. coli O157:H7 EDL933 to sublethal concentrations (1, 0.1, and 0.01 μg/mL) of common bAGPs, including monensin, tylosin, chlortetracycline, and oxytetracycline. Because the bAGP concentrations used in the study were markedly less than the MICs (Table 2), bAGP treatment did not affect the growth of E. coli O157 (data not shown). The propagation of Stx phages was induced significantly by chlortetracycline and oxytetracycline at concentrations as low as 0.01 μg/mL (Figure 1, panel A). Because E. coli O157:H7 EDL933 harbors 2 Stx prophages, BP-933W (stx2) and CP-933V (stx1) (24), the level of stx2 expression was specifically measured with an stx2::gfp fusion construct to determine the propagation level of Stx2 phage. Consistently, bAGP treatment substantially increased the level of stx2 expression (Figure 1, panel B). Because the primary mechanism for antibiotic-mediated induction of phage propagation is the SOS response, we also determined the level of recA expression after exposure to bAGPs. Consistent with the changes in the level of Stx phage propagation (Figure 1, panels A, B), chlortetracycline and oxytetracycline significantly induced recA expression (Figure 1, panel C). Of note, chlortetracycline and oxytetracycline induced Stx phage propagation and stx2 expression at levels similar to those of ciprofloxacin, a DNA-damaging antibiotic frequently used as a phage inducer (Figure 1).
Increased Propagation of Stx Phages in Bovine STEC Strains by bAGPs
When we further examined Stx phage induction by bAGPs with 3 stx2+/stx1– E. coli strains from cattle, we found that exposure to a sublethal concentration (0.1 μg/mL) of chlortetracycline and oxytetracycline significantly induced the propagation of Stx2 phage in the bovine STEC isolates, whereas 0.1 µg/mL of monensin did not induce phage propagation, and 0.1 μg/mL of tylosin exhibited strain-dependent variations in the phage induction (Figure 2).
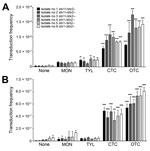
Transfer of Stx Phages in Bovine E. coli Isolates by Sublethal Concentrations of bAGPs
We determined the frequency of Stx2 phage transfer with 6 stx2-negative E. coli isolates from cattle, including 3 stx1+/stx2– E. coli strains and 3 stx1–/stx2– E. coli strains, by using a detoxified EDL933 derivative in which stx2 was replaced with a kanamycin resistance cassette. The donor E. coli (EDL933 Δstx2::Km, a detoxified strain) and the recipient stx2-negative E. coli strains were co-cultivated in the presence of sublethal concentrations (0.01 μg/mL and 0.1 μg/mL) of bAGPs. The recipient bovine stx2-negative E. coli isolates are all sensitive to kanamycin and resistant to β-lactams or tetracycline, and the detoxified EDL933 derivative is resistant to kanamycin and sensitive to β-lactams and tetracycline. Therefore, the Stx phage transfer made the recipient strains resistant to both kanamycin and β-lactams or tetracycline. Subtherapeutic treatment of bAGPs, particularly chlortetracycline and oxytetracycline, substantially enhanced the transfer of Stx2 phage in E. coli (Figure 3, panel A). The transduction rate was slightly increased by 0.1 µg/mL tylosin but not notably affected by 0.1 µg/mL monensin (Figure 3, panel A). When the concentration of bAGPs was reduced to 0.01 μg/mL, tylosin did not mediate the Stx phage transfer. However, chlortetracycline and oxytetracycline significantly mediated the transfer of Stx phage in E. coli even at 0.01 μg/mL (Figure 3, panel B).
Induction of Stx Phage Propagation by Therapeutic Concentrations of Chlortetracycline and Oxytetracycline
Whereas tylosin and monensin are used at low concentrations in cattle feed, chlortetracycline and oxytetracycline are sometimes used at therapeutic levels to prevent infection (6). Thus, we investigated the effects of high concentrations of chlortetracycline and oxytetracycline on the propagation of Stx phages. High concentrations of chlortetracycline significantly increased the propagation of Stx phages in a concentration-dependent manner, whereas the level of Stx phage induction by oxytetracycline is already significantly high at 1 μg/mL in comparison with higher concentrations of oxytetracycline (2–8 μg/mL) and even the highest concentration of chlortetracycline used in the study (8 μg/mL) (Figure 4). These findings demonstrate that therapeutic application of chlortetracycline may enhance the dissemination of Stx phages more significantly than subtherapeutic doses and that oxytetracycline is a highly potent inducer of Stx phage propagation even at low concentrations.
Confirmation of Stx Phage Transfer by bAGPs
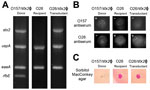
To confirm Stx phage transfer by bAGPs, we randomly chose transductant colonies from the co-culture experiment described earlier (Figure 3) for further verification with PCR to detect genes specific for the Stx2 phage (stx2), E. coli (uspA), E. coli virulence (eaeA), and O157 serotype (rfbEO157) (Figure 5, panel A). Figure 5 shows representative data to exhibit the dissemination of the stx2 gene to stx2-negative E. coli O26 (bovine isolate no. 1 in Figure 3) by exposure to bAGPs. We selected E. coli O26 because this serotype is the most frequently isolated non-O157 STEC (9). Treatment with bAGP changed bovine E. coli O26 from stx2-negative to stx2-positive. We also performed a latex agglutination test to confirm the serotype after transduction (Figure 5, panel B). The capability of sorbitol fermentation in non-O157 strains was confirmed by growing on sorbitol MacConkey agar plates (Figure 5, panel C). The results clearly showed that non-O157 E. coli horizontally acquired stx2 by phage transduction after exposure to bAGPs.
In livestock production, antibiotics are routinely added to feed for growth promotion and disease prevention. Although these AGPs are used at subtherapeutic concentrations, a substantial number of studies have shown that AGPs may negatively affect public health by providing selective pressure to increase antibiotic-resistant pathogens (25,26). Our study showed that, in addition to growing public health concerns about antibiotic resistance, some AGPs may facilitate the transmission of virulence factors in E. coli even at extremely low concentrations. Whereas the effect of monensin and tylosin on the propagation of Stx phages seemed to be marginal, chlortetracycline and oxytetracycline significantly induced the propagation of Stx phages (Figures 1, 2, 4) and mediated the transfer of Stx phages in E. coli (Figure 3).
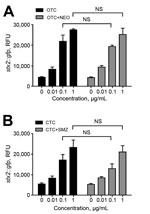
Tetracyclines are widely used in agriculture, accounting for 44% and 37% of marketed agricultural antibiotics in the United States (27) and the European Union (28), respectively. Compared with monensin and tylosin, oxytetracycline and chlortetracycline most significantly affected the transmission of Stx phages, even at concentrations as low as 0.01 μg/mL (Figure 3, panel B). This concentration is substantially lower than concentrations in the large intestines of cattle, which are 0.3 μg/mL after chlortetracycline feeding for growth promotion (70 mg/head/day throughout feedlot period) and 1.7 μg/mL after feeding for disease prevention (350 mg/head/day for 28 days) (29). A previous observational study reported that the percentage of detecting stx-positive commensal E. coli was increased in cattle from 48% to 80% by oxytetracycline injection and chlortetracycline addition to feed (30). Although they did not conclusively say that the increase in stx-positive animals is from oxytetracycline and chlortetracycline treatment in cattle, they suggested that antibiotic treatment may be the reason for the increased prevalence (30). Sometimes, chlortetracycline and oxytetracycline are mixed with other antibiotics, such as neomycin and sulfamethazine, to maintain weight gains and feed efficiency for cattle under stress conditions (4). We observed that tetracycline combinations with these antibiotics induced Stx phage propagation just as comparably as a single treatment of chlortetracycline or oxytetracycline alone (Figure 6), suggesting that oxytetracycline and chlortetracycline are the major bAGPs that induce propagation of Stx phages. Another concern about bAGPs would be associated with poor absorption of orally administered antibiotics in animal guts (31). Approximately 75% of dietary chlortetracycline is excreted in cattle manure without being digested (32), and chlortetracycline is the antimicrobial compound that is most frequently detected in cattle manure at levels as high as 20 mg/kg (33). Given the high residue concentrations in mature, unmetabolized tetracycline residues may also affect the dissemination of Stx phages in cattle manure.
Although use of chlortetracycline and oxytetracycline in cattle is not consistent (4), the levels of SOS response induction and Stx phage propagation by these 2 antibiotics were comparable (Figure 1). In addition to subtherapeutic use in feed, therapeutic concentrations of oxytetracycline and chlortetracycline are sometimes added to feed as metaphylaxis in feedlot cattle (6). We observed that chlortetracycline induced propagation of Stx phages more significantly at high concentrations than at low subtherapeutic concentrations (Figure 4). Surprisingly, propagation of Stx phages by 1 μg/mL oxytetracycline was comparable to that of 8 μg/mL chlortetracycline (Figure 4), suggesting that oxytetracycline is highly effective in phage induction. Possibly, therapeutic administration of chlortetracycline, oxytetracycline, and other antibiotics, particularly those inducing the SOS response and Stx phage propagation (e.g., fluoroquinolones) (12), would significantly affect the spread of Stx phages; however, its effect would be limited because therapeutic antibiotics are usually used to treat disease in individual animals.
Previous studies have reported that antibiotic treatment significantly increases the propagation of Stx phages (24, 34–36). However, little attention has been paid to the effects of nonprescription bAGPs on the transmission of Stx phages in E. coli, although phages are a well-known vehicle for horizontal gene transfer and cattle are the primary reservoirs for E. coli O157:H7. Presumably, the underestimation of bAGPs might result from low concentrations of antibiotics in cattle feed. Nevertheless, in this study, we demonstrated that some bAGPs, particularly chlortetracycline and oxytetracycline, are implicated in the diversification of stx-positive O serotypes in E. coli by facilitating the horizontal transfer of Stx phages even at substantially low concentrations. Thus, use of these agents could lead to emergence of pathogenic E. coli.
Dr. Kim is a postdoctoral research fellow in the School of Public Health at the University of Alberta. His research interests are the pathophysiology and antibiotic resistance of foodborne pathogens, particularly pathogenic E. coli.
Acknowledgments
We thank Shimshon Belkin for providing the plasmid u66recA (recA::egfp).
This study was supported by grant 401843-2012-RGPIN from the Natural Sciences and Engineering Research Council of Canada to B.J. The research infrastructure was supported by the Leaders Opportunity Fund from the Canada Foundation for Innovation. The work of J.S. was supported by the National Basic Research Program of China (2013CB127200).
References
- Organisation for Economic Co-operation Development. Global antimicrobial use in the livestock sector. 2015 [cited 2015 Jul 1]. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=TAD/CA/APM/WP(2014)34/FINAL&docLanguage=En
- Union of Concerned Scientists. Hogging it: estimates of antimicrobial abuse in livestock. 2001 [cited 2015 Jul 1]. http://www.ucsusa.org/food_and_agriculture/our-failing-food-system/industrial-agriculture/hogging-it-estimates-of.html#.VuAuafJf3IU
- United States Department of Agriculture. Feedlot 2011 part IV: health and health management on U.S. feedlots with a capacity of 1,000 or more head. 2013 [cited 2015 Jul 1]. https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_PartIV.pdf
- Giguère S, Prescott JF, Dowling PM. Antimicrobial therapy in veterinary medicine. 5th ed. Ames (IA): Wiley-Blackwell; 2013. p. 495–518.
- Nagaraja TG, Chengappa MM. Liver abscesses in feedlot cattle: a review. J Anim Sci. 1998;76:287–98 .PubMedGoogle Scholar
- Gustafson RH, Kiser JS. Nonmedical uses of the tetracyclines. In: Hlavka JJ, Boothe JH, editors. The tetracyclines. New York: Springer Science; 2012. p. 405–39.
- Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38 .PubMedGoogle Scholar
- Johnson KE, Thorpe CM, Sears CL. The emerging clinical importance of non-O157 Shiga toxin–producing Escherichia coli. Clin Infect Dis. 2006;43:1587–95. DOIPubMedGoogle Scholar
- Gould LH, Mody RK, Ong KL, Clogher P, Cronquist AB, Garman KN, Increased recognition of non-O157 Shiga toxin–producing Escherichia coli infections in the United States during 2000–2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathog Dis. 2013;10:453–60. DOIPubMedGoogle Scholar
- Allison HE. Stx-phages: drivers and mediators of the evolution of STEC and STEC-like pathogens. Future Microbiol. 2007;2:165–74. DOIPubMedGoogle Scholar
- Toshima H, Yoshimura A, Arikawa K, Hidaka A, Ogasawara J, Hase A, Enhancement of Shiga toxin production in enterohemorrhagic Escherichia coli serotype O157:H7 by DNase colicins. Appl Environ Microbiol. 2007;73:7582–8. DOIPubMedGoogle Scholar
- Kimmitt PT, Harwood CR, Barer MR. Toxin gene expression by Shiga toxin–producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis. 2000;6:458–65. DOIPubMedGoogle Scholar
- Davis TK, McKee R, Schnadower D, Tarr PI. Treatment of Shiga toxin–producing Escherichia coli infections. Infect Dis Clin North Am. 2013;27:577–97. DOIPubMedGoogle Scholar
- Nguyen Y, Sperandio V. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front Cell Infect Microbiol. 2012;2:90.
- Etcheverría AI, Padola NL. Shiga toxin–producing Escherichia coli: factors involved in virulence and cattle colonization. Virulence. 2013;4:366–72. DOIPubMedGoogle Scholar
- Callaway TR, Elder RO, Keen JE, Anderson RC, Nisbet DJ. Forage feeding to reduce preharvest Escherichia coli populations in cattle, a review. J Dairy Sci. 2003;86:852–60DOIPubMedGoogle Scholar
- Naylor SW, Low JC, Besser TE, Mahajan A, Gunn GJ, Pearce MC, Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect Immun. 2003;71:1505–12. DOIPubMedGoogle Scholar
- Pruimboom-Brees IM, Morgan TW, Ackermann MR, Nystrom ED, Samuel JE, Cornick NA, Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc Natl Acad Sci U S A. 2000;97:10325–9. DOIPubMedGoogle Scholar
- Sagi E, Hever N, Rosen R, Bartolome AJ, Rajan Premkumar J, Ulber R, Fluorescence and bioluminescence reporter functions in genetically modified bacterial sensor strains. Sens Actuators B Chem. 2003;90:2–8. DOIGoogle Scholar
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–5. DOIPubMedGoogle Scholar
- Paton AW, Paton JC. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602 .PubMedGoogle Scholar
- Chen J, Griffiths MW. PCR differentiation of Escherichia coli from other gram-negative bacteria using primers derived from the nucleotide sequences flanking the gene encoding the universal stress protein. Lett Appl Microbiol. 1998;27:369–71. DOIPubMedGoogle Scholar
- Valdivia RH, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol. 1996;22:367–78. DOIPubMedGoogle Scholar
- Herold S, Siebert J, Huber A, Schmidt H. Global expression of prophage genes in Escherichia coli O157:H7 strain EDL933 in response to norfloxacin. Antimicrob Agents Chemother. 2005;49:931–44. DOIPubMedGoogle Scholar
- Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24:718–33. DOIPubMedGoogle Scholar
- Aarestrup FM. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark: chapter 1: introduction. APMIS. 2000;108:5–6. DOIGoogle Scholar
- US Food and Drug Administration. 2013 Summary report on antimicrobials sold or distributed for use in food-producing animals. 2015 [cited 2015 Jul 1]. http://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM440584.pdf
- European Medicines Agency. Sales of veterinary antimicrobial agents in 26 EU/EEA countries in 2012. 2014 [cited 2015 Jul]. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/10/WC500175671.pdf
- Cazer CL, Volkova VV, Grohn YT. Use of pharmacokinetic modeling to assess antimicrobial pressure on enteric bacteria of beef cattle fed chlortetracycline for growth promotion, disease control, or treatment. Foodborne Pathog Dis. 2014;11:403–11. DOIPubMedGoogle Scholar
- O'Connor AM, Ziebell KA, Poppe C, McEwen SA. Verotoxins in commensal Escherichia coli in cattle: the effect of injectable subcutaneous oxytetracycline in addition to in-feed chlortetracycline on prevalence. Epidemiol Infect. 2004;132:77–85. DOIPubMedGoogle Scholar
- Sarmah AK, Meyer MT, Boxall AB. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–59. DOIPubMedGoogle Scholar
- Elmund GK, Morrison SM, Grant DW, Nevins SM. Role of excreted chlortetracycline in modifying the decomposition process in feedlot waste. Bull Environ Contam Toxicol. 1971;6:129–32. DOIPubMedGoogle Scholar
- Zhao L, Dong YH, Wang H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci Total Environ. 2010;408:1069–75DOIPubMedGoogle Scholar
- McGannon CM, Fuller CA, Weiss AA. Different classes of antibiotics differentially influence Shiga toxin production. Antimicrob Agents Chemother. 2010;54:3790–8. DOIPubMedGoogle Scholar
- Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. Quinolone antibiotics induce Shiga toxin–encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–70. DOIPubMedGoogle Scholar
- Cornick NA, Helgerson AF, Mai V, Ritchie JM, Acheson DW. In vivo transduction of an Stx-encoding phage in ruminants. Appl Environ Microbiol. 2006;72:5086–8. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 22, Number 5—May 2016
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Byeonghwa Jeon, 3-57A South Academic Building, University of Alberta, Edmonton, AB T6G 2G7, Canada
Top