Variable Protease-Sensitive Prionopathy Transmission to Bank Voles
Romolo Nonno
1, Silvio Notari
1, Michele Angelo Di Bari, Ignazio Cali, Laura Pirisinu, Claudia d’Agostino, Laura Cracco, Diane Kofskey, Ilaria Vanni, Jody Lavrich, Piero Parchi, Umberto Agrimi, and Pierluigi Gambetti

Author affiliations: Istituto Superiore di Sanità, Rome, Italy (R. Nonno, M.A. Di Bari, L. Pirisinu, C. d’Agostino, I. Vanni, U. Agrimi); Case Western Reserve University, Cleveland, Ohio, USA (S. Notari, I. Cali, L. Cracco, D. Kofskey, J. Lavrich, P. Gambetti); University of Bologna, Bologna, Italy (P. Parchi); Istituto delle Scienze Neurologiche di Bologna, Bologna (P. Parchi)
Main Article
Figure 6
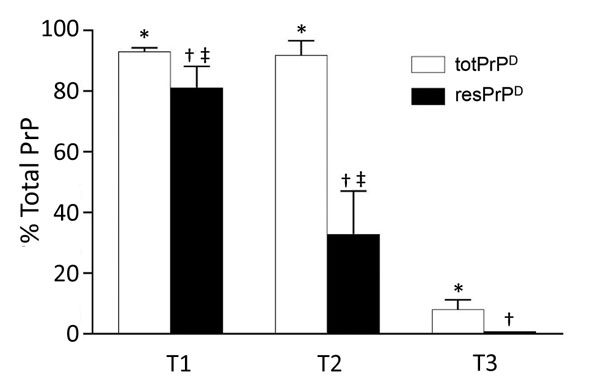
Figure 6. Relative quantities of totPrPD and resPrPD in T1–T3 phenotypes. totPrPD accounted for 93.1% and resPrPD for 81.3% of total PrP recovered from bank voles harboring the T1 phenotype. Corresponding percentages for T2 were 91.0% and 33.0%, and T3 totPrPD and resPrPD accounted only for 8.0% and 0.2% of total PrP and differed significantly from both T1 and T2 in each of the 2 components. ResPrPD also differed significantly between T1 and T2 (each bar represents the mean ± SD of n = 3 T1, n = 3 T2, and n = 5 T3; all data are from bank voles 109I; antibody 9A2). bv, bank vole; resPrPD protease-resistant, disease-related prion protein; totPrPD, comprising protease-sensitive PrPD and resPrPD. *p<0.0001; †p<0.0001 vs. T1 and p<0.05 vs. T2; ‡p<0.01.
Main Article
Page created: December 18, 2018
Page updated: December 18, 2018
Page reviewed: December 18, 2018
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
