Volume 26, Number 1—January 2020
Synopsis
Candidatus Mycoplasma haemohominis in Human, Japan
Figure 2
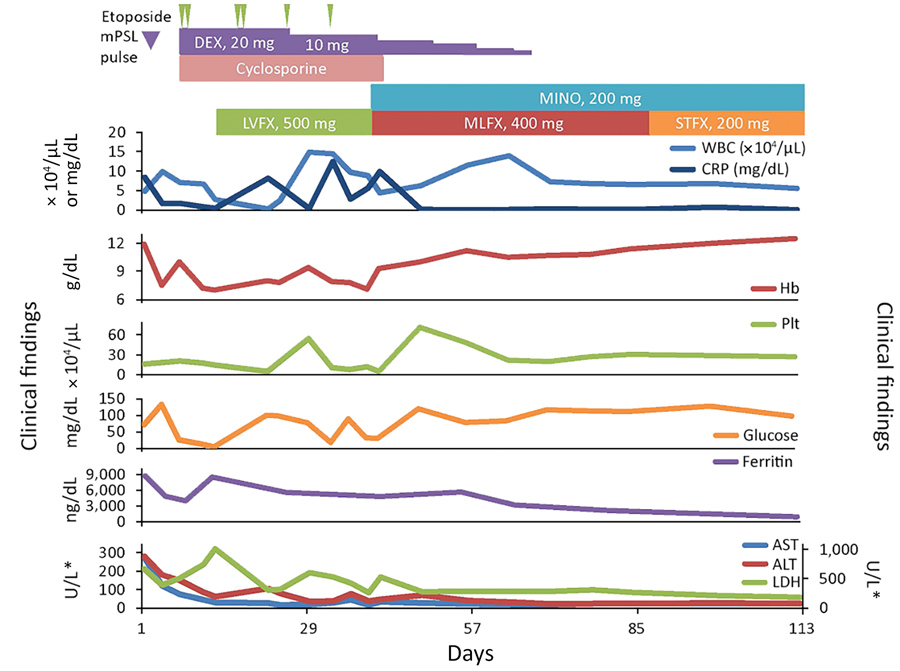
Figure 2. Clinical course for a 42-year-old man infected with Candidatus Mycoplasma haemohominis, Japan. *For ALT, AST, and LDH, left y-axis is for AST and ALT and right y-axis is for LDH. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; DEX, dexamethasone; Hb, hemoglobin; LDH, lactate dehydrogenase; LVFX, levofloxacin; MINO, minocycline; MLFX, moxifloxacin; mPSL, methylprednisolone; PLT, platelets; STFX, sitafloxacin; WBC, white blood cells.
1These authors contributed equally to this article.
Page created: December 18, 2019
Page updated: December 18, 2019
Page reviewed: December 18, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.