Volume 27, Number 5—May 2021
Research
Engineered NS1 for Sensitive, Specific Zika Virus Diagnosis from Patient Serology
Figure 4
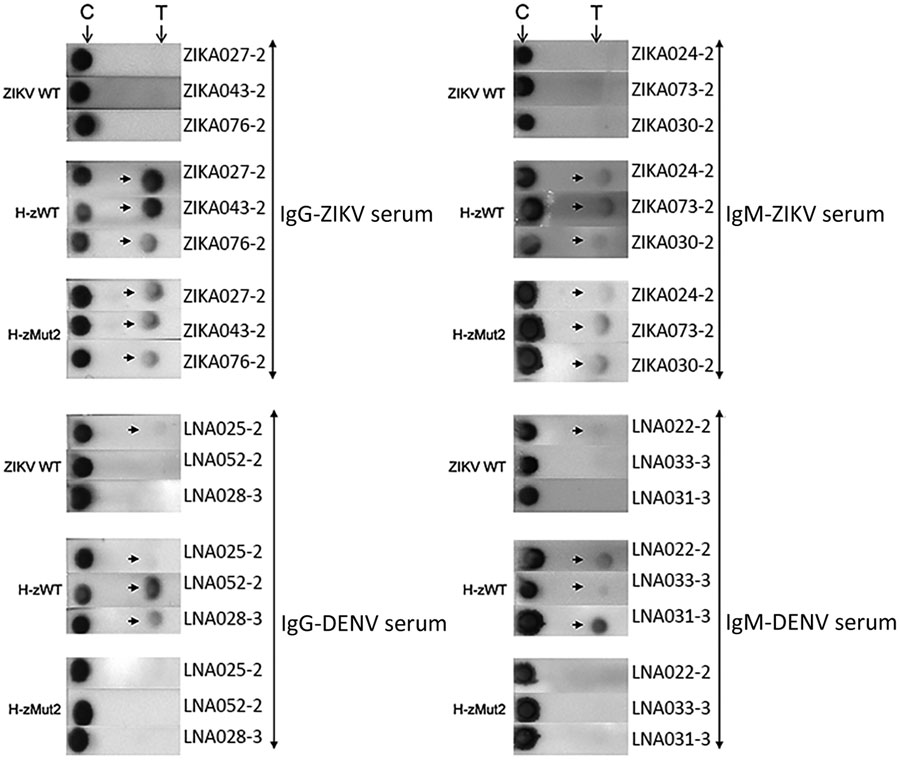
Figure 4. Immunochromatographic assay (IA) of H-zMut2 F1 IA for IgM and IgG detection in study of Zika diagnosis, Singapore. H-zMut2 as capture antigen in the F1 IA format was tested with training set for detecting IgG (left) and IgM (right). Representative strips show a comparison of performance for WT-NS1, H-zWT and H-zMut2. Overall, H-Mut2 showed higher specificity than H-zWT (against DENV plasma, bottom panels), though both H-Mut2 and H-zWT showed greater sensitivity compared to WT-NS1 (against ZIKV plasma, top panels). The arrows indicate positive signals at the test line (T), upstream of the control line (C). DENV, dengue virus; OD, optical density; WT, wild type; ZIKV, Zika virus.
1These authors contributed equally to this article.
Page created: April 05, 2021
Page updated: April 20, 2021
Page reviewed: April 20, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.