Volume 27, Number 5—May 2021
Research
Engineered NS1 for Sensitive, Specific Zika Virus Diagnosis from Patient Serology
Figure 7
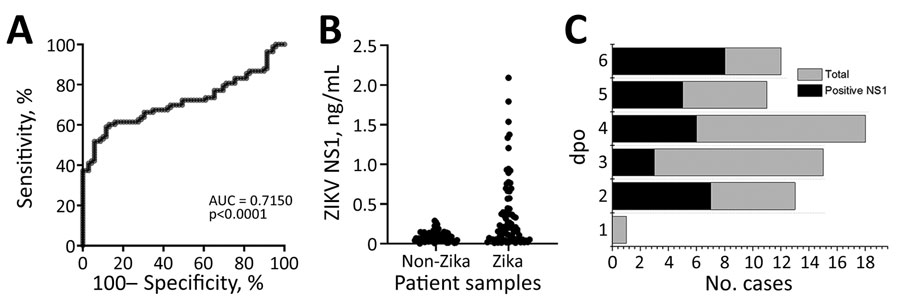
Figure 7. ELISA for ZIKV NS1 detection in study of Zika diagnosis, Singapore. A) Receiver operating characteristics curve analysis showing the performance of C12-C11 sandwich ELISA when tested against ZIKV-infected or non–ZIKV-infected samples. B) ZIKV NS1 quantification in patient samples using in-house antibody pairs. Each dot represents an individual patient sample. C) Distribution of number of plasma cases (x-axis) over dpo (y-axis) for ZIKV NS1 ELISA tested with the validation set; positive plasma (black) and the total plasma cases (gray) at each dpo are also shown. DENV, dengue virus; dpo, days postonset of symptoms; NS1, nonstructural protein 1; ZIKV, Zika virus.
1These authors contributed equally to this article.
Page created: April 05, 2021
Page updated: April 20, 2021
Page reviewed: April 20, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.