Volume 32, Number 1—January 2026
Research
Enhanced Isolation and Detection of COVID-19 in Hospitalized Patients Undergoing Antiviral Therapy
Figure 1
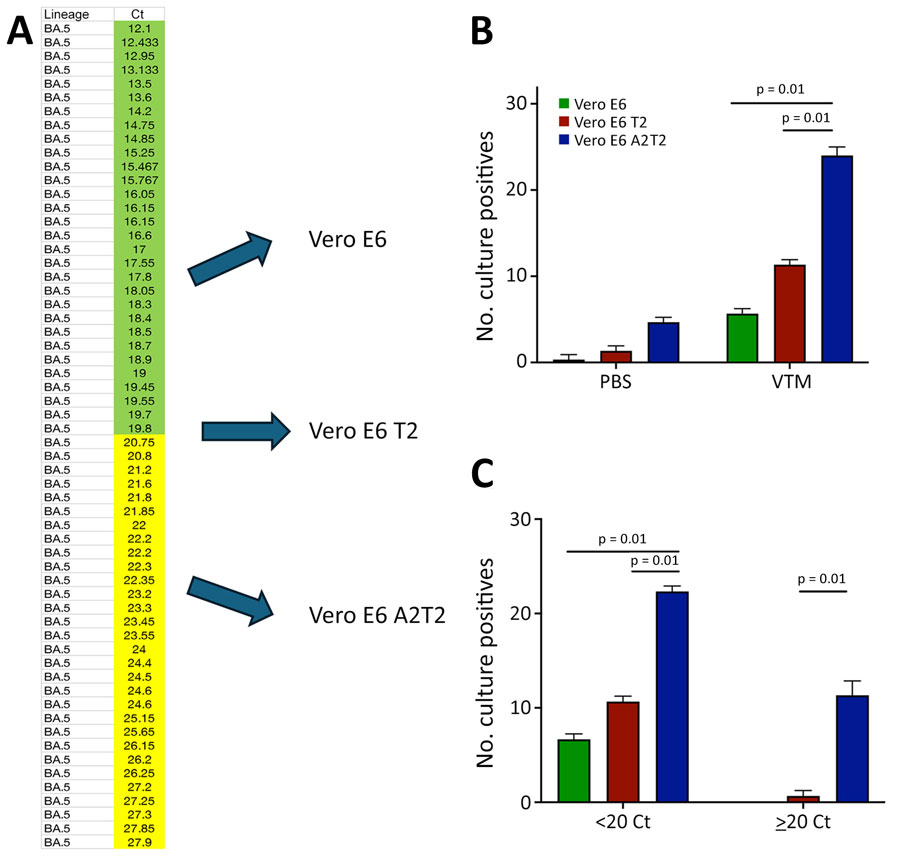
Figure 1. Comparative analysis of SARS-CoV-2 infectious virus isolation using Vero E6–derived cell lines from study on the enhanced isolation and detection of COVID-19 in hospitalized patients undergoing antiviral therapy. A) Thirty nasal swab specimens confirmed by real time PCR to contain the SARS-CoV-2 BA.5 variant, representing a range of Ct values from 12.1–27.9, were inoculated in triplicate onto Vero E6, Vero E6 T2, and Vero E6 A2T2 cell lines. B) Number of BA.5-positive nasal swab specimens collected in either PBS or VTM and inoculated in triplicate onto the 3 cell lines. C) BA.5-positive nasal swab specimens collected in VTM and stratified by Ct values; samples with values <20 or >20 were inoculated in triplicate into the 3 cell lines, and the number of successful virus isolations was plotted with corresponding means +SD. Error bars indicate SDs. Ct, cycle threshold; PBS, phosphate-buffered saline; Vero E6 T2, Vero E6 cells expressing transmembrane protease serine 2; Vero E6 A2T2, Vero E6 cells expressing both transmembrane protease serine 2 and angiotensin-converting enzyme 2; VTM, viral transport medium.