Volume 21, Number 1—January 2015
Research
Protocol for Metagenomic Virus Detection in Clinical Specimens1
Figure 2
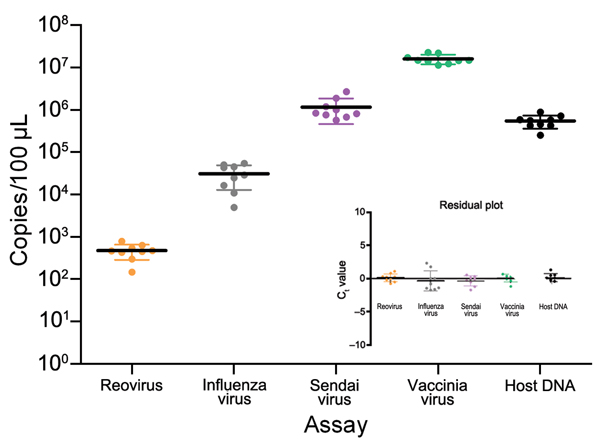
Figure 2. Validation of test aliquots of infected mode used for development of tissue-based universal virus detection for viral metagenomics protocol. Every ninth aliquot was extracted, and viral copy numbers were determined by using a quantitative PCR. Standard deviations (error bars), medians (solid horizontal lines), and residual plots indicate homogeneity and mixture of test specimens. Ct, cycle threshold.
1Preliminary results of this study were presented at the Biodefense and Emerging Infectious Diseases Meeting, January 29, 2014, Washington DC, USA.
Page created: January 05, 2015
Page updated: January 05, 2015
Page reviewed: January 05, 2015
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.