Volume 21, Number 1—January 2015
Research
Protocol for Metagenomic Virus Detection in Clinical Specimens1
Figure 6
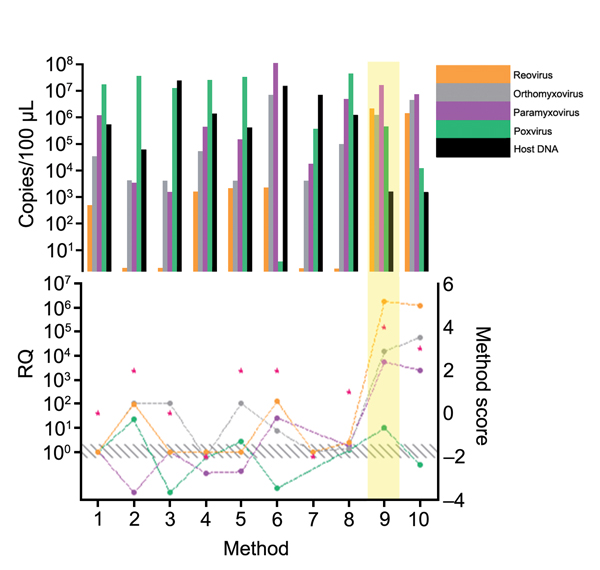
Figure 6. Comparison of extraction methods used for development of tissue-based universal virus detection for viral metagenomics protocol. Copy numbers were measured by quantitative PCR in duplicate. RQ, relative quantification: RQ (2 – ΔΔCt); (ΔΔCt = Δ purified – Δ unprocessed). Lower panel left y-axis indicates signal-to-noise ratio (RQ) for all viruses tested. The method with the highest score was used to establish the protocol and is shaded in yellow. Red stars indicate highest scores. Diagonally striped area indicates not significant. Ct, cycle threshold. Numbers along baseline indicate method used. 1, Nucleospin RNA II (Macherey Nagel, Dueren, Germany); 2, Nucleospin DNA (Macherey Nagel); 3, RTP DNA/RNA Virus Ultra Sense (Invitek, Berlin Germany); 4, RTP DNA/RNA Virus Mini Kit (Invitek); 5, QIAmp UltraSens Virus Kit (QIAGEN, Hilden, Germany); 6, Viral Mini Kit (QIAGEN); 7, QIAmp MinElute Virus Spin Kit (QIAGEN); 8, PureLink Viral RNA/DNA (Invitrogen Life Technologies, Grand Island, NY, USA); 9, TRIzol LS; 10, phenol chloroform.
1Preliminary results of this study were presented at the Biodefense and Emerging Infectious Diseases Meeting, January 29, 2014, Washington DC, USA.