Volume 23, Number 1—January 2017
Research
Modeling Tool for Decision Support during Early Days of an Anthrax Event
Figure 3
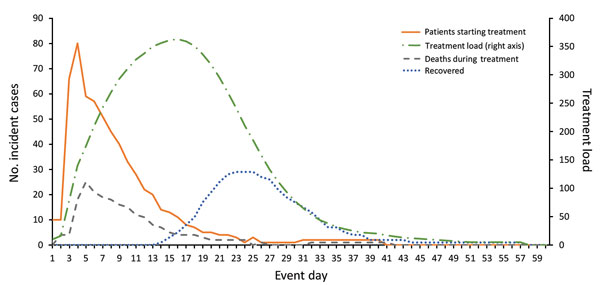
Figure 3. Projected daily patients seeking treatment, daily treatment load, and treatment outcomes by event day (baseline scenario). Estimates were calculated by using values shown in Table 2. Base case scenario is the same as PEP Evaluation Scenario 3 (practical) (Table 3). PEP, postexposure prophylaxis.
1These senior authors contributed equally to this article.
Page created: December 14, 2016
Page updated: December 14, 2016
Page reviewed: December 14, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.