Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients
Nisreen M.A. Okba
1, Marcel A. Müller
1, Wentao Li
1, Chunyan Wang, Corine H. GeurtsvanKessel, Victor M. Corman, Mart M. Lamers, Reina S. Sikkema, Erwin de Bruin, Felicity D. Chandler, Yazdan Yazdanpanah, Quentin Le Hingrat, Diane Descamps, Nadhira Houhou-Fidouh, Chantal B.E.M. Reusken, Berend-Jan Bosch, Christian Drosten, Marion P.G. Koopmans, and Bart L. Haagmans

Author affiliations: Erasmus Medical Center, Rotterdam, the Netherlands (N.M.A. Okba, C.H. GeurtsvanKessel, M.M. Lamers, R.S. Sikkema, E. deBruin, F.D. Chandler, C.B.E.M. Reusken, M.P.G. Koopmans, B.L. Haagmans); Charité-Universitätsmedizin Berlin, Berlin, Germany (M.A. Müller, V.M. Corman, C. Drosten); German Centre for Infection Research, Berlin (M.A. Müller, V.M. Corman, C. Drosten); Utrecht University, Utrecht, the Netherlands (W. Li, C. Wang, B.-J. Bosch); Université de Paris, Paris, France (Y. Yazdanpanah, Q. Le Hingrat, D. Descamps); Hôpital Bichat-Claude Bernard, Paris (Y. Yazdanpanah, Q. Le Hingrat, D. Descamps, N. Houhou-Fidouh); RIVM, Bilthoven, the Netherlands (C.B.E.M. Reusken)
Main Article
Figure 5
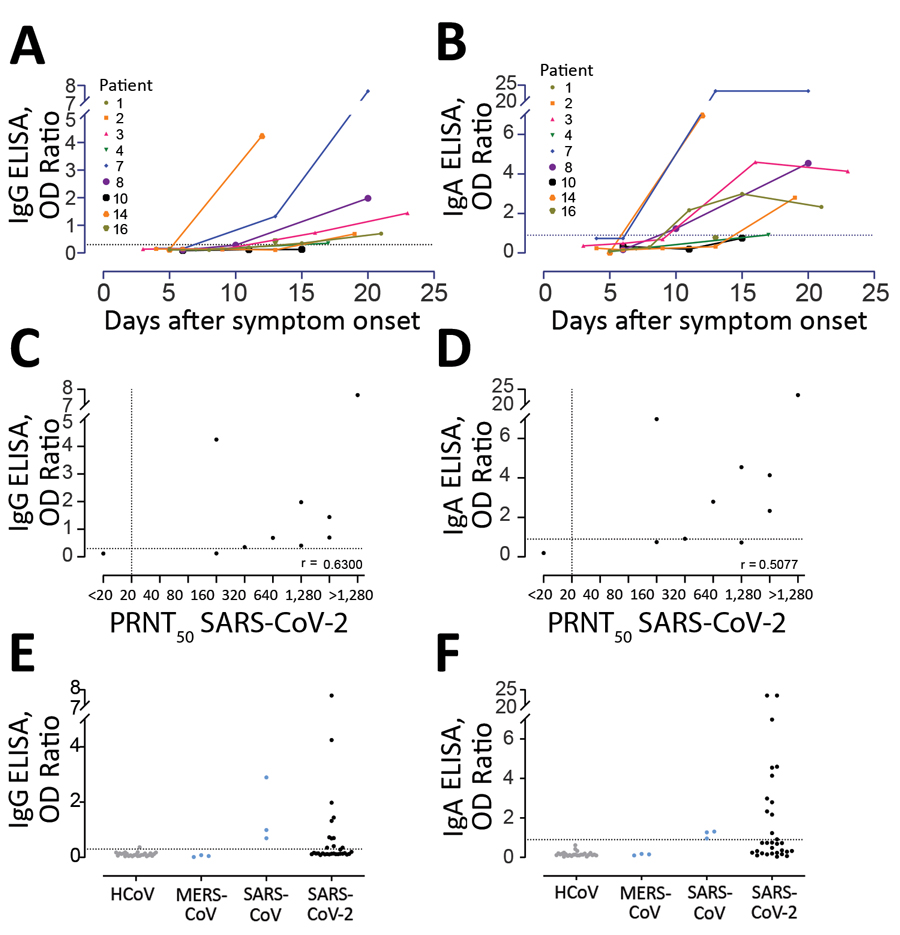
Figure 5. Sensitivity of 2 commercial ELISAs for detection of SARS-CoV-2 specific IgG (A, C, E) and IgA (B, D, F). A, B) Kinetics of antibody responses in 9 COVID-19 patients from Germany; C, D) correlation between antibody responses detected by the ELISAs and the plaque reduction neutralization assay; E, F) kits were tested for specificity by using 18 serum samples from patients infected with HCoV (4 from patients infected with HCoV-229E, 3 from patients infected with HCoV-HKU1, 4 from patients infected with HCoV-NL63, and 7 from patients infected with HCoV-OC43), MERS-CoV (n = 3), and SARS-CoV (n = 3). Dotted horizontal lines indicate ELISA cutoff values. COVID-19, coronavirus disease 2019; HCoV, human coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; N, nucleocapsid; OD, optical density; PRNT50, plaque reduction neutralization assay; RBD, receptor-binding domain; RFU, relative fluorescence unit; S, spike; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2; severe acute respiratory syndrome coronavirus 2.
Main Article
Page created: April 08, 2020
Page updated: June 18, 2020
Page reviewed: June 18, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
