Volume 30, Number 7—July 2024
Research
Sialic Acid Receptor Specificity in Mammary Gland of Dairy Cattle Infected with Highly Pathogenic Avian Influenza A(H5N1) Virus
Figure 2
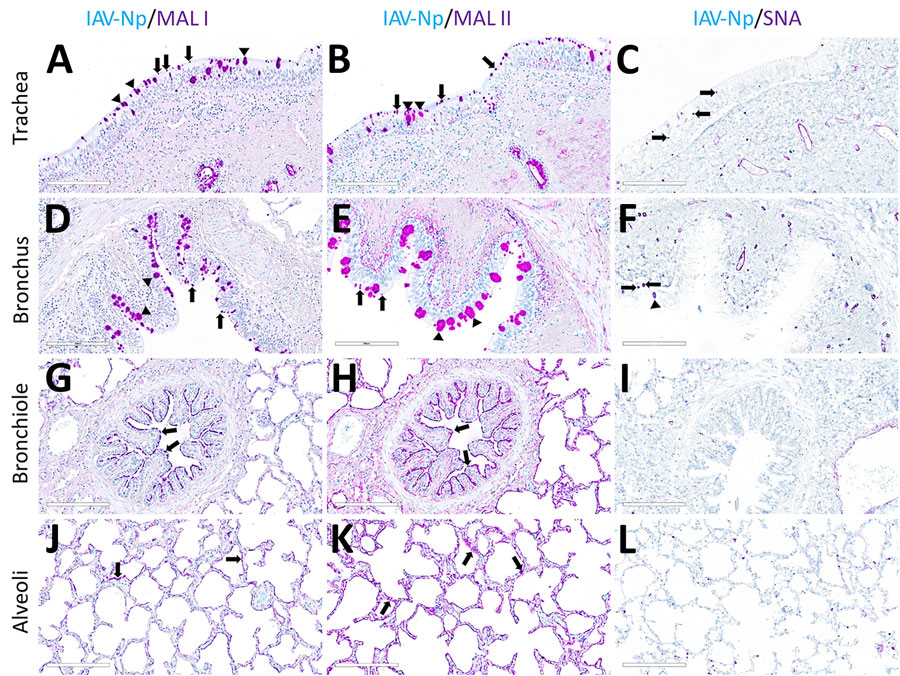
Figure 2. Respiratory tract tissues from a US dairy cow infected with highly pathogenic avian influenza A(H5N1) virus, showing IAV-Np (teal chromogen), individually duplexed with MAL-I (magenta chromogen), MAL-II (magenta chromogen), and SNA (magenta chromogen) using chromogenic staining. Representative images are shown for IAV-Np/MAL-I (A, D, G, J), IAV-Np/MAL-II (B, E, H, K), and IAV-Np/SNA (C, F, I, L) are shown. No IAV-Np was observed in the unaffected respiratory tissue sections. Intense granular to punctate labeling for MAL-I (A) and MAL-II (B) were observed within goblet cells (arrowheads), along the apical ciliated margin (arrows), and glands of the trachea. SNA labeling (C) was confined to intraepithelial round cells (arrows), endothelium, and glands of the trachea. Similar labeling for MAL-I (D) and MALII (E) was observed within the bronchial lumen within goblet cells (arrowheads) and along the apical cell margin (arrows). SNA (F) labeling was only observed in rare goblet cells (arrowheads) and lamina proprial round cells (arrows) in the bronchus. Bronchioles had diffuse, fine, fibrillary to apical membranous labeling (arrows) MAL-I (G) and MALII (H). No substantial labeling was detected within the mucosal epithelial cells of bronchioles with SNA (I). Diffuse, fine, apical membranous labeling of pneumocytes lining alveoli was observed with MAL-I (J) and MAL-II (K) (arrows). SNA (L) labeling within the alveolar portions of the lung was confined to endothelium and interstitial round cells. Scale bars indicate 200 μm. IAV-Np, influenza A virus nucleoprotein; MAL, Maackia amurensis lectin; SNA, Sambucus nigra lectin.
1These first authors contributed equally to this article.