Volume 30, Number 8—August 2024
Research
Scrapie versus Chronic Wasting Disease in White-Tailed Deer
Cite This Article
Citation for Media
Abstract
White-tailed deer are susceptible to scrapie (WTD scrapie) after oronasal inoculation with the classical scrapie agent from sheep. Deer affected by WTD scrapie are difficult to differentiate from deer infected with chronic wasting disease (CWD). To assess the transmissibility of the WTD scrapie agent and tissue phenotypes when further passaged in white-tailed deer, we oronasally inoculated wild-type white-tailed deer with WTD scrapie agent. We found that WTD scrapie and CWD agents were generally similar, although some differences were noted. The greatest differences were seen in bioassays of cervidized mice that exhibited significantly longer survival periods when inoculated with WTD scrapie agent than those inoculated with CWD agent. Our findings establish that white-tailed deer are susceptible to WTD scrapie and that the presence of WTD scrapie agent in the lymphoreticular system suggests the handling of suspected cases should be consistent with current CWD guidelines because environmental shedding may occur.
Prion diseases, or transmissible spongiform encephalopathies, result from the misfolding of a host’s endogenous prion protein and the accumulation of the misfolded form of the prion protein (PrPSc) (1). Accumulation of PrPSc is associated with neurodegeneration and spongiform lesions that invariably kill the host (1). Prion diseases affect mammals: scrapie in sheep, chronic wasting disease (CWD) in cervids, bovine spongiform encephalopathy in cattle, and Creutzfeldt-Jakob disease in humans (2). The hallmark of transmissible spongiform encephalopathies is that the misfolded protein itself, devoid of the nucleic acid that drives viruses and bacteria (1), is able to transmit prion disease between animals (3).
CWD was first identified in mule deer and black-tailed deer in 1967 (4). CWD has spread in cervids across North America and has been detected internationally (5). Within a species, prion diseases can occur as strains. Phenotypic features that can be used to differentiate strains may include host susceptibility based on prion protein sequence, incubation periods, age at clinical onset, tissue tropism, histologic patterns of PrPSc accumulation, biochemical and biological properties of PrPSc, and range of susceptible species (6–9). Strain properties can be further differentiated by using rodent models (10). Although species outside the cervid family are susceptible to CWD (11–17), there is no evidence that the disease has been transmitted to humans (18).
Speculation about the origin of CWD has often implicated the classical scrapie agent of sheep (19–22), which is effectively transmitted to white-tailed deer intracranially and oronasally (23–25). That experimental disease, hereafter referred to as WTD scrapie, is lymphotropic (23,24), which means it is associated with environmental contamination and horizontal transmission (26–28) and could enable spread of the WTD scrapie agent in the cervid population (29–31).
Our purpose with this study was to examine the potential for white-tailed deer to transmit the WTD scrapie agent to other deer via oronasal exposure and to compare the disease phenotype to that of the CWD agent. We discovered that although differences exist between the WTD scrapie agent and the CWD agent in white-tailed deer, the presence of lymphoid involvement suggests that environmental contamination is highly likely. As the geographic distribution and disease incidence of CWD in white-tailed deer increases, information about the potential role of scrapie in the burgeoning CWD epidemic could assist in mitigation efforts. Our animal experiment was approved by the National Animal Disease Center Institutional Animal Care and Use Committee.
Our study population comprised 3 white-tailed deer that were homozygous for glutamine at codon 95 and glycine at codon 96 (QQ95/GG96) of the PRNP gene. To enable comparison of WTD scrapie with CWD by the assays used in this study, we used samples from a deer experimentally inoculated with the CWD agent (National Animal Disease Center [NADC] identification [ID] 1548); the deer was of the same genotype and inoculated by the same route as the 3 deer in our study population.
We oronasally inoculated deer with 1 mL of a 10% wt/vol brainstem homogenate in phosphate-buffered saline (PBS) from a white-tailed deer (NADC ID 18, publication ID 9 [23]) in which classical scrapie had developed (isolate no. 13-7 ARQ/ARQ [32]) after oronasal inoculation. We conducted antemortem rectal biopsies 17 months after inoculation. Animal caretakers observed the deer daily and euthanized them when they exhibited clinical signs (e.g., weight loss, hair loss, excessive salivation, diarrhea, and progressive weakness). We performed necropsies on the euthanized deer and collected the following samples: whole brain, cerebrospinal fluid, brainstem, spinal cord (cervical, thoracic, and lumbar segments), dorsal root ganglia, eyes, turbinate, nerves (optic, trigeminal, sciatic), lymph nodes (retropharyngeal, prescapular, mesenteric, popliteal), thymus, thyroid gland, trachea, esophagus, foregut (rumen, reticulum, omasum, abomasum), jejunum, ileum, cecum, recto-anal mucosa–associated lymphoid tissue, kidney, adrenal gland, liver, urine, spleen, lung, skin, and muscles (tongue, masseter, heart, diaphragm, triceps brachii, biceps femoris, psoas major, bladder). We collected 2 sets of tissue samples, froze 1 set, and collected the other in 10% formalin and embedded it in paraffin wax. We stained or immunolabelled embedded samples for microscopy and immunohistochemistry. Frozen samples of brainstem and retropharyngeal lymph node underwent enzyme immunoassay. We performed Western blots on brainstem, retropharyngeal lymph node, and cerebrum samples and further evaluated brainstem samples by dot blot PrPSc conformational stability assay and mouse bioassay.
For genotyping of the white-tailed deer, we extracted DNA before conducting PCR. We combined the DNA samples with PCR mix (5× buffer, deoxynucleotide triphosphates, primer #1, primer #2, dimethyl sulfoxide, Herculase II Fusion DNA polymerase [https://www.agilent.com], and double-distilled water) in a thermal cycler (Applied Biosystems, https://www.thermofisher.com) as previously described (33). We modified the program slightly from those previously described: 95°C for 5 minutes, 40 cycles of 95°C for 20 seconds, 54°C for 20 seconds, 72°C for 1 minutes, followed by 72°C for 7 minutes, and held at 4°C until samples were removed. We purified the PCR products by using Amicon Ultra Filters (30 kDa) (Sigma Aldrich, https://www.sigmaaldrich.com) according to manufacturer instructions. We ran the samples on an agarose gel (1%) and DNA sequenced them.
The enzyme immunoassay kit that we used is commercially available (HerdChek, IDEXX Laboratories Inc., https://www.idexx.com), and we followed manufacturer instructions to screen for PrPSc in the cerebrum, brainstem, retina, and retropharyngeal lymph nodes. We determined the negative cutoff threshold by using the negative control provided in the kit. We considered values above the optical density threshold positive. We quantified the misfolded prion protein in brainstem samples by making 2-fold dilutions to compare relative prion loads for cervidized mouse bioassays (Table 1).
We processed frozen tissues as 20% wt/vol protein homogenates by using PBS for Western blotting in a Bead Mill 24 homogenizer (Fisher Scientific; https://www.fishersci.com). Samples were digested by proteinase K (PK; 1 mg/mL) (Invitrogen, https://www.thermofisher.com) for 1 hour at 37°C with agitation (500 rpm), followed by reaction neutralization with Pefabloc (100 mg/mL) (Roche Diagnostics GmbH, https://www.roche.de) and incubation for 20 minutes at room temperature. We completed immunodetection of the misfolded prion protein on the cerebrum (frontal cortex), cervical spinal cord, retina, and retropharyngeal lymph nodes. Because of lack of tissue availability of the brainstem at the obex, we used cervical spinal cord. We prepared samples with lithium dodecyl sulfate sample buffer and 2-mercaptoethanol before loading them onto commercial-grade 12% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) gel and running for 45 minutes at 200 V in MOPS SDS running buffer (Invitrogen) with NuPageTM Antioxidant (Invitrogen) in the center chamber. We then transferred the gel to a polyvinylidene difluoride membrane and blocked with 3% bovine serum albumin in Tris-buffered saline with 0.05% Tween 20. We then probed blots with mouse monoclonal antibodies against the prion protein: 6H4, SHA31, 12B2, and P4, all at 1:10,000 dilution (0.1 μg/mL). The C-terminal antibody 6H4 recognizes amino acids PrP-Ov 148-156/PrP-Bov 155-163 (Prionics, https://www.prionics.com), and the SHA31 C-terminal antibody recognizes amino acids 144–155 (Bertin Bioreagent, https://www.bertin-bioreagent.com) of the prion protein. On the N-terminal of the prion protein, antibody 12B2 targets amino acids PrP-Ov 89-107/PrP-Bov 97-115 (Wageningen Bioveterinary Research, https://www.wur.nl) and antibody P4 targets amino acids PrP-Ov 93-99 (R-Biopharm AG, https://r-biopharm.com). We used an antimouse biotinylated sheep secondary antibody at a 1:400 dilution (Cytiva, https://www.cytivalifesciences.com) and a conjugated streptavidin-horseradish peroxidase at a 1:10,000 dilution (Cytiva) for amplification and signal detection. We incubated primary antibodies overnight in 4°C and the subsequent antibodies for 1 hour each at room temperature. Visualization was achieved by using electrochemiluminescence (Thermo Fisher Scientific, https://www.thermofisher.com and an iBright 1500 (Invitrogen). We used PageRuler Plus prestained protein ladder (Thermo Fisher Scientific) to demark relative weights.
For immunohistochemistry, we used 4-micrometer paraffin-embedded tissue sections, stained the tissues with hematoxylin and eosin, and used an automated Ventana Discovery XT staining machine (Roche Diagnostics, https://diagnostics.roche.com) for microscopic analysis of misfolded prion protein staining. After deparaffinization and rehydration, we treated samples with 98% formic acid for 5 minutes and then performed antigen retrieval at 121°C for 20 minutes by using Diva Decloaker (Biocare Medical, https://biocare.net). We then probed tissue sections with the primary antibody F99/97 and took images with a Nikon Eclipse 55i microscope (https://www.nikonusa.com) by using Infinity Analyze software (Lumera, https://www.lumenera.com).
We completed bioassays in cervidized mice (Tg12 [34]) that received inoculum from the brainstem of deer 1 (WTD scrapie P2; n = 15). For comparison, we inoculated brainstem from first-passage WTD scrapie agent into cervidized mice and derived from the same deer used for inoculation in this study (WTD scrapie P1; n = 25). We inoculated another group of cervidized mice with brainstem from a white-tailed deer with CWD (WTD CWD, n = 9); the mice were anesthetized and intracranially inoculated with 20 μL of 1% wt/vol brainstem homogenate in PBS. After inoculation, we monitored the mice for clinical signs, then euthanized and necropsied them until study completion.
We conducted a conformational stability assay of the misfolded prion protein by using 96-well plates and 5–50 μg of tissue homogenates from white-tailed deer. We denatured tissue homogenates in 0–5.5 M guanidine hydrochloride G7294 (GdnHCl) (Sigma Aldrich, https://www.sigmaaldrich.com) at room temperature for 1 hour. We then filtered the samples on Amersham Protran nitrocellulose membrane (Cytiva) with a Bio-Dot Microfiltration apparatus (Bio-Rad Laboratories, https://www.bio-rad.com), followed by 2 PBS washes and air drying of the membrane for 1 hour. We then incubated them with PK (5 μg/mL) in cell lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 0.5% Igepal CA-630 [https://www.sigmaaldrich.com]) for 1 hour at 37°C. We inactivated digestion with PK with 2 mM phenylmethylsulfonyl fluoride. Denaturation of the membrane took 10 minutes in 3 M guanidine thiocyanate in Tris-HCl (pH 7.8) at room temperature. After 4 PBS washes, we blocked membranes with 5% nonfat milk in in Tris-buffered saline with 0.05% TWEEN 20 for 1 hour, then probed at 4°C overnight with SHA31 (Bertin Technologies, https://www.bertin-technologies.fr) diluted 1∶5,000, followed by horseradish peroxidase–conjugated goat antimouse IgG secondary antibody. We used ECL Plus (Pierce ECL Plus Western Blotting Substrate [Thermo Fisher Scientific]) to develop the membranes, a ChemiDoc imager (Bio-Rad) to take the images, and AzureSpot Pro analysis software (Azure Biosystems, https://azurebiosystems.com) to complete the signal analysis. We completed analysis on 3 biological replicates. We normalized absolute densitometric values by defining the smallest mean of each sample as 0 and largest mean as 1. To produce denaturation curves, we plotted relative levels of the undenatured PrPSc, referred to as Fapp (apparent fractional change of unfolded PrPSc), as a function of GdnHCl concentration. We used a nonlinear least-square 4-parameter sigmoidal dose-response regression with the half maximal denaturation concentration, [GdnHCl]1/2, calculated by using Graphpad Prism software (https://www.graphpad.com). We used the Student t-test to assess the statistical significance of [GdnHCl]1/2.
Of the 3 wild-type (QQ95/GG96) white-tailed deer oronasally inoculated with brainstem homogenate from a deer that succumbed to no. 13-7 classical scrapie, all either exhibited clinical signs (excessive salivation, hair loss, and weight loss) and were euthanized or found dead 21–25.8 months after inoculation (Table 2). Enzyme immunoassays performed on central nervous and lymphoreticular system tissues (Table 2) indicated that all 3 deer were positive for PrPSc in the brainstem. Deer 1 (optical density [OD] 4.00) and 2 (OD 3.26) had relatively more PrPSc, indicated by greater optical density than in deer 3 (OD 1.79) in the brainstem. Regardless, all white-tailed deer had 4.0 PrPSc in the retropharyngeal lymph nodes. Only deer 1 was positive in the cerebrum and retina (OD 4.00).
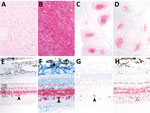
Immunohistochemistry indicated spongiform lesions and misfolded prion protein accumulation in the dorsal motor nucleus of the vagus in the brainstem at the level of the obex in all 3 white-tailed deer inoculated with WTD scrapie (Figure 1, panels A, B). PrPSc accumulation was also detected in the palatine tonsils and retropharyngeal lymph nodes of each deer (Figure 1, panels C, D). Only deer 1 exhibited strong immunolabelling for misfolded prion protein in the retina (Figure 1, panel G). That deer also had the greatest level of spongiform lesions and misfolded prion protein accumulation in the dorsal motor nucleus of the vagus in the brainstem at the level of the obex. The PrPSc in the retina of deer 1 was abundant in the retinal ganglion cells (Figure 1, panel G), similar to that in the retinas of white-tailed deer (Figure 1, panel F) and sheep (Figure 1, panel E) inoculated with the no. 13-7 classical scrapie isolate from sheep. That finding differs from that of white-tailed deer with CWD, in which the retinal ganglion cells generally lack that type of accumulation (Figure 1, panel H). Deer 2 and 3 exhibited minimal immunolabelling for PrPSc in the retina (Table 2). Because staining was limited to the optic disk and plexiform layers in those deer, evaluation of retinal ganglion cells for PrPSc could not be completed.
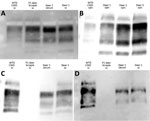
Molecular profile differences were reported for tissues from white-tailed deer with first-passage WTD scrapie because brainstem was CWD-like (relatively higher kDa) and cerebrum was scrapie-like (relatively lower kDa) (23). Western blots were performed to evaluate whether the molecular profile differences would persist. Epitope mapping using different antibodies enabled assessment of approximate PK cleavage sites. When we used C-terminal antibodies (6H4 or SHA31), the molecular profile of the tissues from second-passage WTD scrapie was similar to that of the inoculum as well as tissues from white-tailed deer with CWD (Figure 2, panels A, B). When we used N-terminal antibody 12B2, the inoculum was nonreactive, but CWD and second-passage WTD scrapie isolates appeared similarly reactive (Figure 2, panel C). However, antibody P4 recognized an epitope further toward the N-terminal than 12B2, enough to distinguish between CWD and second-passage WTD scrapie isolates. Although the signal from white-tailed deer CWD cervical spinal cord remained strong, signals from WTD scrapie isolates were either greatly reduced or completely absent when probed with P4 (Figure 2, panel D).
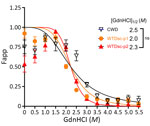
To identify potential strain differences, we inoculated cervidized mice (Tg12) with brainstem material (Table 1). We compared incubation periods of Tg12 mice inoculated with second-passage WTD scrapie agent (WTD scrapie P2; n = 15) with incubation periods of those inoculated with brainstem material from first-passage WTD scrapie agent (WTD scrapie P1; n = 25) and CWD agent. The incubation period in mice inoculated with brainstem from deer 1 (WTD scrapie P2) was similar to that in mice inoculated with WTD scrapie P1 (Figure 3). The average incubation time in mice inoculated with WTD scrapie P2 was 322 days after inoculation and did not differ significantly from that in mice inoculated with WTD scrapie P1 (340 days after inoculation). The incubation periods in mice inoculated with either WTD scrapie P1 or P2 differed significantly (p<0.0001) from those in mice inoculated with CWD agent, for which average incubation period was 199 days after inoculation (WTD CWD, n = 9). Attack rates for the 3 cohorts of mice were high (94%–100%). We performed conformational stability assays to determine if phenotypic differences between WTD scrapie passages and CWD were associated with differences in resistance of PrPSc to increasing concentrations of denaturant. After denaturation by guanidine hydrochloride, there was no difference in the conformational stability of the misfolded prion protein from the cervical spinal cords of white-tailed deer with second-passage WTD scrapie, first-passage WTD scrapie, or CWD (Figure 4). Therefore, molecular and mouse bioassay differences are not associated with differences in the conformational stability of PrPSc.
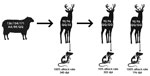
Our study demonstrates that the WTD scrapie agent can be efficiently transmitted to wild-type white-tailed deer (Figure 5). After oronasal inoculation with the WTD scrapie agent, all 3 wild-type (QQ95/GG96) white-tailed deer displayed clinical signs and were positive for PrPSc in multiple nervous and lymphoreticular tissues (100% attack rate). Spongiform lesions, PrPSc accumulation, and molecular phenotypes of second-passage WTD scrapie were similar to those of the WTD scrapie inoculum. White-tailed deer are susceptible to infection with scrapie agents from various sources (23–25,35). Even when white-tailed deer at the lowest risk for CWD infection (SS96) (33,36,37) were exposed to classical sheep scrapie, they all succumbed to the disease (23–25). Unlike the initial passage of WTD scrapie agent in white-tailed deer (23), all brain tissues from our study exhibited a consistent molecular profile, probably because of the WTD scrapie agent stabilizing on the white-tailed deer PrP and differential neural prion selection (38). Further studies are needed to investigate the role that PRNP polymorphisms play in the disease progression of WTD scrapie in white-tailed deer (8,39,40).
WTD scrapie remains different from CWD on second passage in white-tailed deer. WTD scrapie differs from CWD in that WTD scrapie PrPSc accumulates in the retinal ganglion cells (Figure 1, panels E–G), has a shorter PK-resistant core (Figure 2, panels C, D), and has longer incubation periods in mice (Figure 3). Those differences did not result from differences in genotype (41), PrPSc conformational stability (Figure 4), or relative quantity of PrPSc in the inoculum (Table 1, 1% wt/vol) (42). Although many CWD strains in cervids have been characterized (5), none are able to address the longstanding hypothesis that classical sheep scrapie may be the origin of CWD in cervids (43). Our evidence suggests that WTD scrapie differs from CWD in white-tailed deer. Nevertheless, our evidence is limited to 2 experimental passages and the genotypes of deer used for those passages because genotype can affect prion transmission characteristics (7,8,25,44). Evaluating how the scrapie agent evolves in white-tailed deer requires subsequent passages in white-tailed deer of varying genotypes.
WTD scrapie has not been detected in wild or farmed cervids. If WTD scrapie were to be detected in cervids, management would remain consistent with current measures for CWD. WTD scrapie, like CWD, is lymphotropic. Lymphotropism occurs early in disease progression before neuroinvasion and indicates that the animal is shedding PrPSc into its environment and contaminating it (26–28). Although the WTD scrapie agent propagates effectively on white-tailed deer PrP, the only reported cases have been through experimental exposure. Because of the National Scrapie Eradication Program in 2001, cases of classical scrapie in farmed sheep have dramatically dropped and no case of classical scrapie has detected in the United States since January 2021 (https://www.aphis.usda.gov/sites/default/files/scrapie-quarterly-report-june-2024.pdf). The potential for zoonoses of cervid-derived PrPSc is still not well understood (6,18,45–47); however, interspecies transmission can increase host range and zoonotic potential (48–50). Therefore, to protect herds and the food supply, suspected cases of WTD scrapie should be handled the same as cases of CWD.
Dr. Lambert is an assistant professor at Des Moines University. Her main research interest is prion disease.
Acknowledgment
This research was supported in part by an appointment to the Agricultural Research Service Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy (DOE) and the US Department of Agriculture. ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract no. DE-SC0014664. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of the US Department of Agriculture, DOE, or ORAU/ORISE.
References
- Lambert ZJ, Greenlee JJ, Cassmann ED, West Greenlee MH. Differential accumulation of misfolded prion strains in natural hosts of prion diseases. Viruses. 2021;13:2453. DOIPubMedGoogle Scholar
- Miller MW, Wild MA. Epidemiology of chronic wasting disease in captive white-tailed and mule deer. J Wildl Dis. 2004;40:320–7. DOIPubMedGoogle Scholar
- Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. DOIPubMedGoogle Scholar
- Otero A, Duque Velasquez C, McKenzie D, Aiken J. Emergence of CWD strains. Cell Tissue Res. 2023;392:135–48. DOIPubMedGoogle Scholar
- Pritzkow S, et al. North American and Norwegian chronic wasting disease prions exhibit different potential for interspecies transmission and zoonotic risk. J Infect Dis. 2021.PubMedGoogle Scholar
- Otero A, Duque Velásquez C, Johnson C, Herbst A, Bolea R, Badiola JJ, et al. Prion protein polymorphisms associated with reduced CWD susceptibility limit peripheral PrPCWD deposition in orally infected white-tailed deer. BMC Vet Res. 2019;15:50. DOIPubMedGoogle Scholar
- Johnson CJ, Herbst A, Duque-Velasquez C, Vanderloo JP, Bochsler P, Chappell R, et al. Prion protein polymorphisms affect chronic wasting disease progression. PLoS One. 2011;6:
e17450 . DOIPubMedGoogle Scholar - Moore J, Tatum T, Hwang S, Vrentas C, West Greenlee MH, Kong Q, et al. Novel strain of the chronic wasting disease agent isolated from experimentally inoculated elk with LL132 prion protein. Sci Rep. 2020;10:3148. DOIPubMedGoogle Scholar
- Fraser H, Dickinson AG. Scrapie in mice. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J Comp Pathol. 1973;83:29–40. DOIPubMedGoogle Scholar
- Cassmann ED, Frese RD, Greenlee JJ. Second passage of chronic wasting disease of mule deer to sheep by intracranial inoculation compared to classical scrapie. J Vet Diagn Invest. 2021;33:711–20. DOIPubMedGoogle Scholar
- Bartz JC, Marsh RF, McKenzie DI, Aiken JM. The host range of chronic wasting disease is altered on passage in ferrets. Virology. 1998;251:297–301. DOIPubMedGoogle Scholar
- Moore SJ, West Greenlee MH, Kondru N, Manne S, Smith JD, Kunkle RA, et al. Experimental transmission of the chronic wasting disease agent to swine after oral or intracranial inoculation. J Virol. 2017;91:e00926–17. DOIPubMedGoogle Scholar
- Greenlee JJ, Nicholson EM, Smith JD, Kunkle RA, Hamir AN. Susceptibility of cattle to the agent of chronic wasting disease from elk after intracranial inoculation. J Vet Diagn Invest. 2012;24:1087–93. DOIPubMedGoogle Scholar
- Hamir AN, Miller JM, Kunkle RA, Hall SM, Richt JA. Susceptibility of cattle to first-passage intracerebral inoculation with chronic wasting disease agent from white-tailed deer. Vet Pathol. 2007;44:487–93. DOIPubMedGoogle Scholar
- Hamir AN, Kunkle RA, Miller JM, Greenlee JJ, Richt JA. Experimental second passage of chronic wasting disease (CWD(mule deer)) agent to cattle. J Comp Pathol. 2006;134:63–9. DOIPubMedGoogle Scholar
- Race B, Meade-White KD, Phillips K, Striebel J, Race R, Chesebro B. Chronic wasting disease agents in nonhuman primates. Emerg Infect Dis. 2014;20:833–7. DOIPubMedGoogle Scholar
- Zink RM. Genetic and evolutionary considerations of the Chronic Wasting Disease - Human species barrier. Infect Genet Evol. 2020;84:
104484 . DOIPubMedGoogle Scholar - LaFauci G, Carp RI, Meeker HC, Ye X, Kim JI, Natelli M, et al. Passage of chronic wasting disease prion into transgenic mice expressing Rocky Mountain elk (Cervus elaphus nelsoni) PrPC. J Gen Virol. 2006;87:3773–80. DOIPubMedGoogle Scholar
- Meyerett-Reid C, Wyckoff AC, Spraker T, Pulford B, Bender H, Zabel MD. De novo generation of a unique cervid prion strain using protein misfolding cyclic amplification. MSphere. 2017;2:e00372–16. DOIPubMedGoogle Scholar
- Tamgüney G, Miller MW, Giles K, Lemus A, Glidden DV, DeArmond SJ, et al. Transmission of scrapie and sheep-passaged bovine spongiform encephalopathy prions to transgenic mice expressing elk prion protein. J Gen Virol. 2009;90:1035–47. DOIPubMedGoogle Scholar
- Greenlee JJ, Moore SJ, Cassmann ED, Lambert ZJ, Kokemuller RD, Smith JD, et al. White-tailed deer are susceptible to the agent of classical sheep scrapie after experimental oronasal exposure. J Infect Dis. 2023;227:1386–95. DOIPubMedGoogle Scholar
- Greenlee JJ, Smith JD, Kunkle RA. White-tailed deer are susceptible to the agent of sheep scrapie by intracerebral inoculation. Vet Res (Faisalabad). 2011;42:107. DOIPubMedGoogle Scholar
- Angers R, Christiansen J, Nalls AV, Kang HE, Hunter N, Hoover E, et al. Structural effects of PrP polymorphisms on intra- and interspecies prion transmission. Proc Natl Acad Sci U S A. 2014;111:11169–74. DOIPubMedGoogle Scholar
- John TR, Schätzl HM, Gilch S. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion. 2013;7:253–8. DOIPubMedGoogle Scholar
- Henderson DM, Denkers ND, Hoover CE, McNulty EE, Cooper SK, Bracchi LA, et al. Progression of chronic wasting disease in white-tailed deer analyzed by serial biopsy RT-QuIC and immunohistochemistry. PLoS One. 2020;15:
e0228327 . DOIPubMedGoogle Scholar - Davenport KA, Christiansen JR, Bian J, Young M, Gallegos J, Kim S, et al. Comparative analysis of prions in nervous and lymphoid tissues of chronic wasting disease-infected cervids. J Gen Virol. 2018;99:753–8. DOIPubMedGoogle Scholar
- Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol. 2015;89:9338–47. DOIPubMedGoogle Scholar
- Tennant JM, Li M, Henderson DM, Tyer ML, Denkers ND, Haley NJ, et al. Shedding and stability of CWD prion seeding activity in cervid feces. PLoS One. 2020;15:
e0227094 . DOIPubMedGoogle Scholar - Mammadova N, Cassmann E, Greenlee JJ. Successful transmission of the chronic wasting disease (CWD) agent to white-tailed deer by intravenous blood transfusion. Res Vet Sci. 2020;133:304–6. DOIPubMedGoogle Scholar
- Moore SJ, Smith JD, Greenlee MH, Nicholson EM, Richt JA, Greenlee JJ. Comparison of two US sheep scrapie isolates supports identification as separate strains. Vet Pathol. 2016;53:1187–96. DOIPubMedGoogle Scholar
- Haley N, Donner R, Merrett K, Miller M, Senior K. Selective breeding for disease-resistant PRNP variants to manage chronic wasting disease in farmed whitetail deer. Genes (Basel). 2021;12:1396. DOIPubMedGoogle Scholar
- Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, et al. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci. 2005;25:7944–9. DOIPubMedGoogle Scholar
- Madsen-Bouterse SA, Schneider DA, Zhuang D, Dassanayake RP, Balachandran A, Mitchell GB, et al. Primary transmission of chronic wasting disease versus scrapie prions from small ruminants to transgenic mice expressing ovine or cervid prion protein. J Gen Virol. 2016;97:2451–60. DOIPubMedGoogle Scholar
- Haley NJ, Merrett K, Buros Stein A, Simpson D, Carlson A, Mitchell G, et al. Estimating relative CWD susceptibility and disease progression in farmed white-tailed deer with rare PRNP alleles. PLoS One. 2019;14:
e0224342 . DOIPubMedGoogle Scholar - Seabury CM, Lockwood MA, Nichols TA. Genotype by environment interactions for chronic wasting disease in farmed US white-tailed deer. G3 (Bethesda). 2022;12:jkac109.
- Wagner K, Pierce R, Gordon E, Hay A, Lessard A, Telling GC, et al. Tissue-specific biochemical differences between chronic wasting disease prions isolated from free-ranging white-tailed deer (Odocoileus virginianus). J Biol Chem. 2022;298:
101834 . DOIPubMedGoogle Scholar - Hwang S, Greenlee JJ, Vance NM, Nicholson EM. Source genotype influence on cross species transmission of transmissible spongiform encephalopathies evaluated by RT-QuIC. PLoS One. 2018;13:
e0209106 . DOIPubMedGoogle Scholar - Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM, McKenzie D. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J Gen Virol. 2006;87:2109–14. DOIPubMedGoogle Scholar
- Duque Velásquez C, Kim C, Haldiman T, Kim C, Herbst A, Aiken J, et al. Chronic wasting disease (CWD) prion strains evolve via adaptive diversification of conformers in hosts expressing prion protein polymorphisms. J Biol Chem. 2020;295:4985–5001. DOIPubMedGoogle Scholar
- Cassmann ED, Brown QL, Frese AJ, Lambert ZJ, Greenlee MHW, Greenlee JJ. Effect of inoculation with prion dilutions within the dynamic range of ELISA absorbance on prion incubation period. Vet Res Commun. 2022;46:1377–80. DOIPubMedGoogle Scholar
- Ness A, Aiken J, McKenzie D. Sheep scrapie and deer rabies in England prior to 1800. Prion. 2023;17:7–15. DOIPubMedGoogle Scholar
- Duque Velásquez C, Kim C, Herbst A, Daude N, Garza MC, Wille H, et al. Deer prion proteins modulate the emergence and adaptation of chronic wasting disease strains. J Virol. 2015;89:12362–73. DOIPubMedGoogle Scholar
- Wang Z, Qin K, Camacho MV, Cali I, Yuan J, Shen P, et al. Generation of human chronic wasting disease in transgenic mice. Acta Neuropathol Commun. 2021;9:158. DOIPubMedGoogle Scholar
- Hannaoui S, Zemlyankina I, Chang SC, Arifin MI, Béringue V, McKenzie D, et al. Transmission of cervid prions to humanized mice demonstrates the zoonotic potential of CWD. Acta Neuropathol. 2022;144:767–84. DOIPubMedGoogle Scholar
- Race B, Baune C, Williams K, Striebel JF, Hughson AG, Chesebro B. Second passage experiments of chronic wasting disease in transgenic mice overexpressing human prion protein. Vet Res (Faisalabad). 2022;53:111. DOIPubMedGoogle Scholar
- Padilla D, Béringue V, Espinosa JC, Andreoletti O, Jaumain E, Reine F, et al. Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog. 2011;7:
e1001319 . DOIPubMedGoogle Scholar - Joiner S, Asante EA, Linehan JM, Brock L, Brandner S, Bellworthy SJ, et al. Experimental sheep BSE prions generate the vCJD phenotype when serially passaged in transgenic mice expressing human prion protein. J Neurol Sci. 2018;386:4–11. DOIPubMedGoogle Scholar
- Herbst A, Velásquez CD, Triscott E, Aiken JM, McKenzie D. Chronic wasting disease prion strain emergence and host range expansion. Emerg Infect Dis. 2017;23:1598–600. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: July 16, 2024
1Current affiliation: Des Moines University, Des Moines, Iowa, USA.
Table of Contents – Volume 30, Number 8—August 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Justin J. Greenlee, National Animal Disease Center, ARS, USDA, 1920 Dayton Ave, PO Box 70, Ames, IA 50010, USA
Top