Volume 31, Number 3—March 2025
Dispatch
Simultaneous Detection of Sarcocystis hominis, S. heydorni, and S. sigmoideus in Human Intestinal Sarcocystosis, France, 2021–2024
Cite This Article
Citation for Media
Abstract
To elucidate the epidemiology of Sarcocystis spp. parasites in human intestinal infections, we used high-throughput sequencing to investigate human intestinal sarcocystosis cases identified by microscopy in France during 2021–2024. Our results indicate that humans are a definitive host of S. sigmoideus parasites and that occurrence of multiple species in 1 patient is common.
The coccidian parasite Sarcocystis is one of the most frequently identified protozoa of warm-blooded and poikilothermic animals worldwide, causing an intestinal infection in the definitive host or an extraintestinal infection in the intermediate host (1). Human intestinal sarcocystosis (i.e., humans as the definitive host) is rarely reported (2–6); only 3 of nearly 200 described Sarcocystis species have been identified as responsible for human intestinal infections (1). Infection is acquired by ingesting raw or undercooked meat containing cysts of the parasite, such as pork for S. suihominis or beef for S. hominis and S. heydorni (1,7). One human case involving S. cruzi infection was also reported, but the presence of this species, for which canids are the definitive host, has yet to be confirmed in humans (2,8).
Genetic characterization of Sarcocystis spp. is commonly based on the mitochondrial cytochrome c oxidase subunit I (COI) gene sequence. Recently, Rubiola et al. described a new species that infects bovine muscle, named S. sigmoideus (9). Retrospective analyses of genomic data available in GenBank revealed that this species previously had been detected in 2 other carcasses in Italy and 6 in Belgium (10–12). The definitive host for this new species was still unknown (9). Here, we report the presence of S. sigmoideus sporocysts in feces from several human patients in France. We also report cases of S. heydorni infections and highlight a high frequency of patients infected with multiple species simultaneously.
According to the French Ministry of Health, data for these patients were collected as part of routine surveillance and epidemiologic investigations by the National Reference Center for Cryptosporidiosis, Microsporidia and Other Digestive Protozoa (Public Health Code Article L 1413-3, https://www.santepubliquefrance.fr/a-propos/nos-principes-fondateurs/centres-nationaux-de-reference-pour-la-lutte-contre-les-maladies-transmissibles-cnr). Therefore, this study is exempt from institutional review board review.
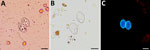
The patients included in this study underwent testing for intestinal parasites during October 2021–July 2024 because of gastrointestinal disorders or for systematic screening. Testing was performed in medical analysis laboratories by microscopic examination of fresh homogenized stool samples highlighting Sarcocystis spp. oocysts, sporocysts, or both (Figure 1). Oocysts/sporocysts were observed in 19 patients (Table), 8 women and 11 men, ranging in age from 19 to 94 years, all living in France. Of the 19 patients, 17 had reported acute, chronic, or occasional diarrhea lasting up to several months; the remaining 2 patients (case identification nos. S01-05 and S01-14) had infection diagnosed during systematic screening. No apparent cause other than Sarcocystis infection has been reported to explain the gastrointestinal disorders, except in 2 patients, 1 with concomitant Salmonella infection (case identification no. S01-03) and 1 with concomitant Taenia saginata infection (case identification no. S01-19). Some other symptoms were occasionally observed, such as abdominal pain, constipation, weight loss, nausea, ileitis, eosinophilia, or blood in stool (Table).
A total of 23 stool samples from the 19 patients were prospectively sent to the French National Reference Center for Cryptosporidiosis, Microsporidia and Other Digestive Protozoa for further molecular analysis (Appendix). In brief, high-throughput sequencing (HTS) was performed on a 332-bp region of the mitochondrial cytochrome c oxidase subunit I gene. We constructed a phylogenetic tree on the basis of the partial gene sequence from the 41 characterized Sarcocystis spp. isolates and reference sequences from GenBank by using the neighbor-joining method (Figure 2). The different Sarcocystis spp. contigs clustered with the reference sequences with a maximum variation of 2 nt over the 332-bp sequence analyzed (Table). Most (11/19) patients were co-infected with multiple Sarcocystis species (S. hominis was most frequently detected); S. sigmoideus infection was detected in 9 patients and S. heydorni infection in 5 patients.
Among the Sarcocystis parasite species present in beef meat (i.e., cattle as intermediate host), S. hominis was the first species reported to infect humans as the definitive host (8). Then, S. heydorni was indirectly considered to infect humans after it was observed in calves fed with sporocysts from the feces of a human volunteer (7). Our results expand on that previous report of human S. heydorni infection by identifying 5 more human cases. Recently, S. sigmoideus was described as a novel species in bovine carcasses. Felids were hypothesized to be the definitive hosts for this species, whereas identical samples harboring both S. sigmoideus and S. hominis suggested a potential zoonotic role (9). Here, we confirm at least the second hypothesis by reporting that humans are a definitive host of S. sigmoideus.
During the study period, we performed molecular analyses on microscopically positive fecal samples (i.e., detection of oocysts/sporocysts) from 19 patients and detected S. sigmoideus parasites in 9 of them. An association with S. hominis parasites was detected in 6 patients and with S. hominis plus S. heydorni parasites in 3 patients.
A limitation of our study is that we could not microscopically distinguish between sporocysts of different species because most patients were co-infected. We also had to consider that, after ingestion of infected meat, some transient Sarcocystis DNA resulted in HTS reads that were not associated with the sporocysts seen in the fecal samples. However, we excluded that possibility because repeated stools spaced over 3 to 24 days for 3 co-infected patients showed the same species in the same proportions (data not shown). Also, in the 19 cases analyzed, we never detected reads from S. cruzi, which is highly prevalent in beef meat but does not infect humans. To definitively confirm that hypothesis, future attempts should be made to perform single-cell sequencing on sporocysts/oocysts isolated from microscopy or by species-specific labeling with species-specific hybridization probes.
The prevalence of S. sigmoideus parasites in cattle has been reported to be low, but it is likely to be underestimated, as suggested by the number of infected patients (9 of 19) in our study (9). Further molecular studies in cattle and human stool are needed to better estimate the real prevalence of S. sigmoideus parasites.
We used HTS for molecular analysis of Sarcocystis spp. parasites in human stools and found that human intestinal sarcocystosis is mainly caused by multiple species simultaneously (11 of 19 patients were co-infected). That finding is in accordance with recent veterinary data that detected Sarcocystis spp. parasites in 64% of randomly sampled cattle carcasses and mixed infections of up to 3 species simultaneously (including S. sigmoideus and S. hominis) in 25% of intralesional samples and in 5.8% of extralesional samples from carcasses condemned because of the presence of bovine eosinophilic myositis (9,10).
S. hominis parasites are considered mildly pathogenic in humans, whereas S. suihominis infection is more virulent (1). However, data about Sarcocystis pathogenicity are scarce, outdated, and mostly derived from volunteers who ingested experimentally infected meat (1). The pathogenicity of S. heydorni and S. sigmoideus parasites is unknown, and further studies are required to address that issue. In conclusion, our data demonstrate that humans are a definitive host for S. sigmoideus parasites and that intestinal sarcocystosis frequently results from infection with multiple species.
Dr. Moniot is a parasitologist and mycologist working in the University Hospital of Clermont-Ferrand, France. His research interests are focused on the epidemiological investigation of parasitic diseases.
Acknowledgments
The datasets generated and analyzed during the study are available from the corresponding author on reasonable request.
This work was supported by internal laboratory funding. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest. The authors declare that no chatbot or artificial intelligence tool was used for any part of the work.
Author contributions: P.P., C.N., and M.M. conceived and designed the study. M.M., D.C., N.A., M.-F.D., and T.N. collected data. M.M. conducted the literature search and drafted the manuscript. P.C. performed sequencing experimentation. All authors edited and approved the final manuscript.
References
- Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of animals and humans. Boca Raton (FL): CRC Press; 2015.
- Agholi M, Shahabadi SN, Motazedian MH, Hatam GR. Prevalence of enteric protozoan oocysts with special reference to Sarcocystis cruzi among fecal samples of diarrheic immunodeficient patients in Iran. Korean J Parasitol. 2016;54:339–44. DOIPubMedGoogle Scholar
- Agholi M, Taghadosi Z, Mehrabani D, Zahabiun F, Sharafi Z, Motazedian MH, et al. Human intestinal sarcocystosis in Iran: there but not seen. Parasitol Res. 2016;115:4527–33. DOIPubMedGoogle Scholar
- Rubiola S, Civera T, Ferroglio E, Zanet S, Zaccaria T, Brossa S, et al. Molecular differentiation of cattle Sarcocystis spp. by multiplex PCR targeting 18S and COI genes following identification of Sarcocystis hominis in human stool samples. Food Waterborne Parasitol. 2020;18:
e00074 . DOIPubMedGoogle Scholar - Van Den Broucke S, Dorny P, Van Esbroeck M, Bottieau E. Microscopic detection of intestinal Sarcocystis infection diagnosed in international travelers at the Institute of Tropical Medicine, Antwerp, Belgium, from 2001 to 2020. Am J Trop Med Hyg. 2023;109:327–31. DOIPubMedGoogle Scholar
- Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev. 2015;28:295–311. DOIPubMedGoogle Scholar
- Dubey JP, van Wilpe E, Calero-Bernal R, Verma SK, Fayer R. Sarcocystis heydorni, n. sp. (Apicomplexa: Sarcocystidae) with cattle (Bos taurus) and human (Homo sapiens) cycle. Parasitol Res. 2015;114:4143–7. DOIPubMedGoogle Scholar
- Dubey JP, Rosenthal BM. Bovine sarcocystosis: Sarcocystis species, diagnosis, prevalence, economic and public health considerations, and association of Sarcocystis species with eosinophilic myositis in cattle. Int J Parasitol. 2023;53:463–75. DOIPubMedGoogle Scholar
- Rubiola S, Moré G, Civera T, Hemphill A, Frey CF, Basso W, et al. Detection of Sarcocystis hominis, Sarcocystis bovifelis, Sarcocystis cruzi, Sarcocystis hirsuta and Sarcocystis sigmoideus sp. nov. in carcasses affected by bovine eosinophilic myositis. Food Waterborne Parasitol. 2024;34:
e00220 . DOIPubMedGoogle Scholar - Zeng H, Van Damme I, Kabi TW, Šoba B, Gabriël S. Sarcocystis species in bovine carcasses from a Belgian abattoir: a cross-sectional study. Parasit Vectors. 2021;14:271. DOIPubMedGoogle Scholar
- Vangeel L, Houf K, Geldhof P, De Preter K, Vercruysse J, Ducatelle R, et al. Different Sarcocystis spp. are present in bovine eosinophilic myositis. Vet Parasitol. 2013;197:543–8. DOIPubMedGoogle Scholar
- Rubiola S, Civera T, Panebianco F, Vercellino D, Chiesa F. Molecular detection of cattle Sarcocystis spp. in North-West Italy highlights their association with bovine eosinophilic myositis. Parasit Vectors. 2021;14:223. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: February 21, 2025
Table of Contents – Volume 31, Number 3—March 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Philippe Poirier, Service de Parasitologie Mycologie, 58 rue Montalembert, CHU Gabriel Montpied, 63003 Clermont-Ferrand CEDEX 1, France
Top