Volume 31, Number 10—October 2025
Research
Comparative Epidemiology of Salmonella enterica Serovers Paratyphi A and Typhi Causing Enteric Fever, Bangladesh, 2018–2020
Cite This Article
Citation for Media
Abstract
Enteric fever remains a public health challenge. We analyzed data from a cluster-randomized Vi-tetanus toxoid conjugate vaccine trial to compare the epidemiology between Salmonella enterica serovars Paratyphi A, which causes paratyphoid fever, and Typhi, which causes typhoid fever. The overall incidence rate of paratyphoid fever was 27 (95% CI 23–32)/100,000 person-years (PY) and of typhoid fever was 216 (95% CI 198–236)/100,000 PY. We observed the highest incidence for both diseases in children 2–4 years of age: 72 (95% CI 41–117)/100,000 PY for paratyphoid and 887 (95% CI 715–1,088)/100,000 PY for typhoid. Lack of private toilets and safe drinking water were associated with both diseases. Prevalence of multidrug resistance was significantly higher in Salmonella Typhi (20.2%) than in Salmonella Paratyphi A (0.8%) (p<0.001). Our data suggest that integrated control measures targeting water, sanitation, and hygiene measures and bivalent vaccine targeting both pathogens are promising strategies to control both diseases.
Enteric fever is caused by Salmonella enterica serovars Paratyphi A, B, and C, which cause paratyphoid fever, and Typhi, which causes typhoid fever. Globally, an estimated 9.3 million cases and 107,459 deaths related to typhoid and paratyphoid fevers occurred in 2021; they especially affected children living in low- and middle-income countries (1). Although Salmonella Typhi played a major role, Salmonella Paratyphi A constituted 23.3% of enteric fever cases in South Asia countries (1). Preventing enteric fever by improving water, sanitation, and hygiene (WASH) infrastructure and practices remains challenging where resources are constrained, placing great reliance on antimicrobial therapy. Increasing multidrug resistance (MDR), defined as resistance to former first-line drugs including ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole (cotrimoxazole), in Salmonella Typhi is hampering successful therapy (2–5). However, previous studies show that MDR rates declined before the emergence of fluoroquinolone-nonsusceptible Salmonella Paratyphi A and Salmonella Typhi strains that render fluoroquinolone less suitable as first-line therapy prescribed by clinicians (6–11). Furthermore, the increase in azithromycin resistance in some settings is worrisome (12,13). Given the overlapping clinical features of Salmonella Paratyphi A infections, laboratory diagnostics are essential for distinguishing between the pathogens to guide appropriate antimicrobial therapy. Because antimicrobial resistance (AMR) makes treatment more difficult, vaccines and WASH interventions play a vital role in prevention.
World Health Organization (WHO)–approved typhoid conjugate vaccines (TCVs) are available (14–18), and bivalent vaccines targeting both Salmonella Paratyphi A and Salmonella Typhi are in development (19). In this study, we analyzed data from a cluster randomized trial of Vi-tetanus (Vi-TT) toxoid conjugate vaccine, based on the Vi capsular polysaccharide of Salmonella Typhi, in urban Dhaka, Bangladesh (20), to assess epidemiologic features of enteric fever. We conducted this study as part of the original vaccine trial study (protocol no. PR-17115), which received ethical approval from the research review committee and the ethical review committee of icddr,b in Dhaka. No additional ethics submission was required. We obtained informed written consent from legal guardians of child participants and from adult participants.
Study Design and Site
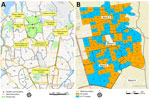
We used the data from a participant- and observer-blind, cluster-randomized, controlled trial of Vi-TT vaccine effectiveness study (20), which was conducted in a densely populated urban area of wards 2, 3, and 5 of Mirpur in Dhaka (Figure 1). Vi-TT was the study vaccine, and the Japanese encephalitis (JE) vaccine was the control vaccine (21). The study area was divided into 150 geographic clusters randomly assigned to the Vi-TT or JE vaccine arms with a 1:1 ratio (20). A baseline census was conducted during February 14–March 25, 2018, to enumerate the entire population in this study area. The census was subsequently updated at 6-month intervals to capture all births, deaths, and migrations. Individual-level and household-level demographic and socioeconomic data, household geopositioning, and WASH data were collected at each census (20).
Passive Surveillance for Enteric Fever
We conducted passive surveillance during April 26, 2018–March 15, 2020, in the 8 main healthcare facilities (HCFs) serving the study population: Mirpur Field Office, Shaheed Suhrawardy Hospital, Radda MCH-FP Centre (Mirpur-10), Mirpur Adhunik Hospital, Kalshi Shishu Hospital, Kurmitola General Hospital, Kingston Hospital, and Aalok Health Care and Hospital Ltd (Figure 1) (20). We enrolled participants of all age groups living in the surveillance catchment area who had a history of fever (>48 hours) or objective fever (axillary temperature >38.0°C) at the time of HCF visit after they or a parent provided written informed consent. We collected blood specimens (3 mL from participants <17 years of age and 5 mL from those >17 years of age) and clinical data upon enrollment. At the time they sought clinical care, we confirmed patient identity either through household identity cards distributed during the census or via census on electronic tablets when identity cards were unavailable.
Laboratory Analysis
We performed microbiologic culture of blood samples using a standard automated BacT/ALERT method (22). When we detected a positive signal from the automated culture machine, we took subcultures on MacConkey agar plates. After overnight incubation at 37°C, we inoculated non–lactose fermenting colonies on Kligler’s Iron Agar (KIA), Motile Indole Urea (MIU), and citrate tubes for biochemical testing to identify Salmonella spp.; we serotyped specimens with Salmonella-specific O and flagellar H antiserum (Denka Sieken, https://www.denka.co.jp) to confirm Salmonella Paratyphi A, B, and C and Salmonella Typhi. We performed antibiotic susceptibility tests (ASTs) for ampicillin, chloramphenicol, trimethoprim/sulfamethoxazole, amoxiclav, azithromycin, cefixime, ceftriaxone, gentamycin, meropenem, ciprofloxacin, and nalidixic acid using the disk diffusion method on antibiotic disks and determined resistance profile in accordance with Clinical and Laboratory Standards Institute guidelines (23).
Definitions
We defined a treatment visit for paratyphoid or typhoid fever as a visit for fever in which the enrolled patient submitted a blood culture positive for Salmonella Paratyphi A or Salmonella Typhi. We concatenated visits for fever in which the onset of symptoms occurred within 14 days of discharge from the previous visit into the same episode. We set the starting date of follow-up as the median date (April 30, 2018) of the baseline vaccination campaign (April 15–May 15, 2018) for unvaccinated patients and the date of actual vaccination for vaccinated patients. For any persons entering our surveillance area (e.g., new births, migration into the study area) after the baseline vaccination campaign, their follow-up period started at the date of birth or date of entry.
Strategies for Analyses
We followed each participant for up to 23 months (April 15, 2018–March 15, 2020) to track the Salmonella Paratyphi A and Salmonella Typhi episodes. We considered participants residing in clusters for both Vi-TT and JE arms for analysis of Salmonella Paratyphi A episodes but considered only those in the JE arm for analysis of Salmonella Typhi episodes. We conducted analyses using both closed cohort and dynamic cohort approaches. We defined the closed cohort as participants who were included in the baseline census or were present at the median date (April 30, 2018) of the baseline vaccination or both. We conducted the closed cohort analysis to evaluate the incidence rates and to measure the associations of disease occurrence with baseline features including sociodemographic and WASH characteristics. We considered only the first episodes for incidence calculation. We measured age-stratified incidence by considering the age of each patient at baseline.
The dynamic cohort referred to the entire population throughout the study period, including those added through birth, immigration after the baseline census, and reentry into the study area after earlier emigration. We conducted dynamic-cohort analysis to provide the best depiction of the disease burden with seasonality, incidence rate (overall and age-stratified), clinical features, and AMR pattern. We calculated seasonality by exploring the monthly incidence of the diseases. For incidence calculation, we considered all episodes detected after starting the follow-up. For age-specific incidence, we used age at follow-up, expressed as age intervals; the numerator was episodes occurring in the population as it passed through the age interval of interest during follow-up, and the denominator was calendar person-time as the population passed through the age interval during follow-up. We conducted descriptive comparative analyses of the clinical features and AMR patterns using the episodes. We did not include follow-up of participants in both cohorts after the dates of death or migration out from the study area or the end of the surveillance (March 15, 2020).
Statistical Analyses
We estimated incidence rates as the total number of Salmonella Paratyphi A or Salmonella Typhi episodes (numerator) divided by the corresponding person-time of follow-up (denominator). In incidence estimation by age or month for seasonality, we considered the numerator as the total episodes from that age or calendar-time period of interest, and the denominator was the total calendar person-time at that age or calendar-time period of interest. We calculated 95% CIs of the incidence rates using the Byar method (24). To identify factors independently associated with the disease, we conducted time-to-event analysis using Cox proportional hazards model, adjusting for age, sex, and design effect. We assessed intracluster correlation (ICC) to investigate the clustering effects on paratyphoid and typhoid fever incidence at the levels of the randomized clusters and households. We calculated ICCs using a generalized linear mixed model under maximum-likelihood estimation via Laplace approximation with the Poisson model as the within-subjects probability model under the repeatability settings (25).
We used χ2 test to measure the associations between categorical variables and Fisher exact test for small (<5) cell frequencies. We assessed continuous variables with t-test or nonparametric Mann-Whitney U test if the data violated distributional assumptions. We considered a 2-tailed test at a 5% level of significance for all statistical analysis. We performed analysis with R statistical software version 4.3.3 (The R Project for Statistical Computing, https://www.r-project.org) using the epiR version 2.0.78 package to estimate the incidence rate and iccCounts for the calculation of ICC.
Study Population and Episodes of Paratyphoid and Typhoid Fever
We enumerated a total of 205,760 participants during the baseline census, of which 102,698 were from the Vi-TT arm and 103,062 were from the JE arm. For the closed cohort, we considered 206,065 participants, 205,760 from the baseline population and 305 new births before the median date of the baseline vaccination. After the subsequent censuses during the study period, we added 120,729 participants, 6,157 from birth and 114,572 from immigration. As a result, we considered a total of 326,794 participants for the dynamic cohort.
Throughout the surveillance of the dynamic cohort, we found 144 episodes of Salmonella Paratyphi A from both the Vi-TT and JE arms and 566 episodes of Salmonella Typhi only from the JE arm. After excluding the episodes that occurred between the end of the study period and the start of follow-up, the total number of Salmonella Paratyphi A cases reported from the dynamic cohort was 121 and the total of Salmonella Typhi episodes was 483. Of the 483 episodes of Salmonella Typhi, 6 were recurrent, whereas all Salmonella Paratyphi A episodes were first episodes. In the closed cohort, the total number of first episodes of Salmonella Paratyphi A was 87 and of Salmonella Typhi was 323. (Figure 2).
Incidence and Seasonality of Paratyphoid and Typhoid Fever
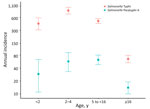
The overall incidence rates (IRs) of paratyphoid for closed and dynamic cohorts were similar, with slightly different CIs: closed cohort IR 27/100,000 PY (95% CI 22–33/100,000 PY); dynamic cohort IR 27/100,000 PY (95% CI 23–32/100,000 PY). Moreover, we observed the highest incidence for both cohorts in children <16 years of age: closed cohort IR 60/100,000 PY (95% CI 46–76/100,000 PY); dynamic cohort IR 57/100,000 PY (95% CI 45–71/100,000 PY). The highest incidence for the closed cohort was among children 2–4 years of age (IR 72/100,000 PY [95% CI 41–117/100,000 PY]) followed by 5 to <16 years (IR 61/100,000 PY [95% CI 45–81/100,000 PY]), <2 years (IR 34/100,000 PY [95% CI 11–80/100,000 PY]), and ≥16 years (IR 12/100,000 PY [95% CI 8–17/100,000 PY]). The highest incidence rate for the dynamic cohort was among children 5 to <16 years of age (IR 62/100,000 PY [95% CI 48–80/100,000 PY]) followed by 2–4 years of age (IR 57/100,000 PY [95% CI 33– 91/100,000 PY]), <2 years of age (IR 29/100,000 PY [95% CI 11– 63/100,000 PY]), and >16 years of age (IR 14/100,000 PY [95% CI 10–19/100,000 PY) (Table 1; Figure 3; Appendix Table 1).
In comparison, the overall incidence rate of typhoid for the closed cohort was 201/100,000 PY (95% CI 180–224/100,000 PY) and for the dynamic cohort 216/100,000 PY (95% CI 198–236/100,000 PY). We observed the highest incidence in children <16 years of age: closed cohort IR 539/100,000 PY (95% CI 478–605/100,000 PY) and dynamic cohort IR 558/100,000 PY (95% CI 504–616/100,000 PY). Among the children <16 years of age, the highest incidence for both cohorts was among children 2–4 years of age; closed cohort IR was 887/100,000 PY (95% CI 715–1,088/100,000 PY) and dynamic cohort IR 863/100,000 PY (95% CI 716–1,031/100,000 PY).
For the closed cohort, the incidence rate among children <2 years of age was 773/100,000 PY (95% CI 573–1,021/100,000 PY), among children 5 to <16 years was 402/100,000 PY (95% CI 340–472/100,000 PY), and among patients >16 years was 45/100,000 PY (95% CI 33–58/100,000 PY). For the dynamic cohort, the incidence rate among children <2 years of age was 430/100,000 PY (95% CI 308–586/100,000 PY), among children 5 to <16 years was 494/100,000 PY (95% CI 433–561/100,000 PY), and among patients >16 years was 65/100,000 PY (95% CI 53–79/100,000 PY). The seasonality of paratyphoid fever incidence was not apparent, whereas typhoid fever peaked in the postmonsoon period (July–August) (Figure 4; Appendix Table 2).
Sociodemographic and WASH Characteristics Related to Paratyphoid and Typhoid Fever
The risk for paratyphoid fever was ≈3 times higher (hazard ratio 2.77 [95% CI 1.65–4.66]; p<0.001) and for typhoid fever 1.5 times higher (hazard ratio 1.55 [95% CI 1.22–1.98]; p<0.001) among members of households having no private toilet compared with members of households that had a private toilet (Tables 2, 3). Moreover, the risk for paratyphoid fever was 2 times higher (hazard ratio 2.1 [95% CI 1.19–3.72]; p = 0.011) and for typhoid 1.4 times higher (hazard ratio 1.4 [95% CI 1.07–1.82]; p = 0.013) higher among members of households having no safe source of drinking water compared with those with a safe source of drinking water. We found a significant clustering effect (ICC 0.00090 [95% CI 0.00014–0.00165]) of typhoid fever at randomized cluster levels, which was considered for the adjustment in the Cox-PH model to evaluate possible risk factors, but found no clustering effect at household cluster levels (Table 4). Moreover, we observed no significant clustering effect of paratyphoid fever at either the household or the randomized cluster levels.
Clinical Characteristics of Paratyphoid and Typhoid Fever
We found no significant differences in clinical features between paratyphoid and typhoid patients, even when stratified by age groups (Table 5). The mean temperature recorded at enrollment for paratyphoid patients was 37.8°C and for typhoid patients, 37.9°C. We observed high fever, defined as an axillary temperature of >40°C, in 2/121 (1.7%) of paratyphoid and 3/483 (0.6%) of typhoid patients when they sought care. Five (4.1%) of 121 paratyphoid patients and 24 (5.0%) of 483 typhoid patients were admitted to a hospital. Of those, 35/121 (28.9%) paratyphoid and 109/483 (22.6%) typhoid patients had a history of antimicrobial drug intake during the 2 weeks before seeking care at the medical facility. Gastrointestinal (abdominal pain, nausea, vomiting, diarrhea) and lower respiratory tract (cough, wheeze, tachypnea) symptoms were equally common among the paratyphoid and typhoid patients. Among paratyphoid patients, 29/121 (24.0%) experienced gastrointestinal symptoms and 33/121 (27.3%) lower-respiratory symptoms; among typhoid patients, 147/483 (30.4%) experienced gastrointestinal symptoms and 130/483 (26.9%) lower-respiratory symptoms. All patients recovered, and none experienced severe complications such as intestinal hemorrhage, perforation, or encephalopathy.
AMR Patterns
One (0.8%) of the 121 Salmonella Paratyphi A isolates was MDR; the rest were susceptible to the first-line antimicrobial drugs ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole. In contrast, 97/480 (20.2%) Salmonella Typhi isolates were MDR (p<0.001 for the difference in occurrence between Salmonella Paratyphi A and Salmonella Typhi). Of note, azithromycin resistance was significantly more frequent (p<0.001) among Salmonella Paratyphi A isolates (14/121 [11.6%]) than among Salmonella Typhi isolates (9/483 [1.9%]). However, we did not observe resistance to any third-generation cephalosporin tested for either pathogen. Almost all Salmonella Paratyphi A (119/121 [98.3%]) and Salmonella Typhi (456/483 [94.4%]) isolates were resistant to nalidixic acid and had intermediate resistance to ciprofloxacin (119/121 [98.3%] Salmonella Paratyphi A and 411/483 [85.1%] Salmonella Typhi isolates) (Table 6).
In an urban slum population in Dhaka, Bangladesh, we identified a moderate incidence of paratyphoid fever (defined as 10–100/100,000 PY) and a high incidence of typhoid fever (defined as >100/100,000 PY) (26). The lack of private toilets and safe drinking water was one of the key risk factors for both diseases. Our findings highlight a high prevalence of both diseases in the population predominantly affecting the same age group and sharing similar risk factors; integrated control and preventive measures might include the use of bivalent vaccines for both typhoid and paratyphoid fevers and improved WASH. We observed a higher burden of MDR in Salmonella Typhi, whereas Salmonella Paratyphi A remained largely susceptible to former first-line drugs but exhibited higher azithromycin resistance. Both pathogens exhibited intermediate resistance to ciprofloxacin.
This study is one of the few detailed comparative epidemiologic investigations on paratyphoid and typhoid fever in Bangladesh undertaken concurrently for both diseases. We found a higher incidence of typhoid fever than paratyphoid fever, consistent with previous studies (1,27,28). The Surveillance for Enteric Fever in Asia Project in 2016–2019 (27) estimated a crude incidence of typhoid fever as 103/100,000 PY and of paratyphoid fever as 16/100,000 PY in Bangladesh. A population-based study under the STRATAA (Strategic Typhoid Alliance across Africa and Asia) consortium also conducted in Dhaka in 2016–2018 (28) showed crude incidences of typhoid as 161/100,000 PY and paratyphoid as 42/100,000 PY. However, during the TCV efficacy study, the incidence of typhoid fever in Nepal (342/100,000 PY) (29) and Bangladesh (213/100,000 PY) (20) was higher than in Malawi (182.7/100,000 PY) (30) in the control group.
Previous studies from Dhaka noted that some environmental factors, such as the preceding rainy season, higher temperature, higher rainfall, or water level of nearby water sources, were associated with a higher incidence of typhoid fever (31,32). Estimating the typhoid prevalence is challenging, given that the factors influencing blood culture positivity can vary widely. Existing studies have shown a strong correlation between enteric fever incidence and poor sanitation and limited access to clean drinking water (33,34) which aligns with our WASH risk factor analysis. Although establishing optimal WASH infrastructure is resource heavy, our findings suggest that public health interventions to improve private toilets and safe drinking water sources should be considered as a near-term investment for tackling both diseases.
The STRATAA study showed the highest reported typhoid incidence in Kathmandu and Dhaka among the 5–9-year age group (28), whereas in this study, we found that children <16 years of age had the highest risk for enteric fever. This finding suggests a bivalent vaccine against both pathogens could be targeted for children <16 years of age. Among children <16 years of age, the highest incidence for both diseases was among children 2–4 years of age, emphasizing that immunization programs with a bivalent vaccine should prioritize infants <2 years of age, as has been done with the implementation of TCV in typhoid-endemic countries (20,29,30).
AMR constitutes a compelling driver for the development of new-generation bivalent vaccines as well as improved WASH interventions. Although first-line antimicrobial drugs remain effective for treating paratyphoid fever in our setting, MDR Salmonella Typhi is highly prevalent. Because the 2 diseases are difficult to distinguish based on clinical features, the selection of appropriate first-line antimicrobial drugs is difficult in many settings (35). Moreover, nalidixic acid resistance and ciprofloxacin intermediate resistance in both pathogens, as well as the substantial level of resistance to azithromycin in Salmonella Paratyphi A, indicate that empiric use of these drugs for treating enteric fever might not be effective in most patients (2,9–11,36). Furthermore, third-generation cephalosporin-resistant Salmonella Typhi has been reported in Pakistan (37). Our study found both organisms to be sensitive to cephalosporin, but Pakistan’s rapid cephalosporin resistance spread signals a need for vigilant monitoring and prudent antimicrobial use.
TCVs have been approved for implementation in the Expanded Program of Immunization of Bangladesh. However, TCVs do not provide cross-protection against Salmonella Paratyphi A. After a period of TCV implementation, the prevalence of paratyphoid fever may increase, perhaps because of strain replacement, as was observed in China with the Vi-polysaccharide typhoid vaccine (38). In aggregate, our findings thus strongly support preventing both diseases by devising effective bivalent vaccines and WASH interventions (19,39,40).
The first limitation of our study was its 2-year duration; seasonality analysis would be strengthened with a longer surveillance period. Second, some cases might have been missed because we enrolled a fraction of eligible participants from the 8 healthcare facilities. Third, the available clinical data were categorized according to systems, not on the basis of individual symptoms or signs, which limited our ability to identify distinguishing clinical features. Fourth, the WASH data was based on a simple questionnaire and could have missed important information. Last, our study was limited to an urban setting in Dhaka, so the findings might not be generalizable to rural areas or other geographic locations. Despite all those limitations, the strength of this study was the prospective, comprehensive, and concurrent surveillance of a large study population for both paratyphoid and typhoid fever, together with repeated censuses, which enabled us to analyze the dynamic cohort in a highly mobile population along with closed cohort from the baseline study population.
In conclusion, we found that, although the incidence of Salmonella Typhi in the study area in Dhaka is greater than that of Salmonella Paratyphi A, the effect of Salmonella Paratyphi A is not negligible, especially in children. Vaccination with a bivalent vaccine should be programmatically feasible given the similar age-specific patterns of incidence, and the similarities of WASH factors associated with the risk for each pathogen suggest that simple WASH interventions might be effective against both pathogens.
Dr. Rahman is an assistant scientist at icddr,b. Her research interests include genomic analysis of enteric pathogens including Salmonella Typhi and Paratyphi to understand the epidemiology, population structure, and antimicrobial resistance pattern.
Acknowledgments
We thank the governments of Bangladesh and Canada for providing unrestricted support. We acknowledge the support from International Vaccine Institute, Kenya Medical Research Institute, and Armauer Hansen Research Institute.
This study was funded by the Bill and Melinda Gates Foundation (grant no. OPP1151153). This publication was made possible through a grant from the Bill & Melinda Gates Foundation (grant no. INV-062435).
Author contributions: J.D.C., F.Q., S.K., and K.Z. contributed to the conception and study design. S.I.A.R, M.G.F., S.E.P., F.K, S.A., and K.H. drafted the manuscript. S.I.A.R., M.G.F., S.E.P., F.K., S.A., K.H., F.A., J.D.C., and X.L. led the analysis and interpretation. J.D.C., F.Q., and A.J.P. supervised and monitored the original study activities. All authors have read and approved the final version of this report and had final responsibility for the decision to submit it for publication.
References
- Institute for Health Metrics and Evaluation (IHME). Global burden of disease 2021: findings from the GBD 2021 study. Seattle (WA): The Institute, 2024.
- Mirza SH, Beeching NJ, Hart CA. Multi-drug resistant typhoid: a global problem. J Med Microbiol. 1996;44:317–9. DOIPubMedGoogle Scholar
- Rowe B, Ward LR, Threlfall EJ. Multidrug-resistant Salmonella typhi: a worldwide epidemic. Clin Infect Dis. 1997;24(Suppl 1):S106–9. DOIPubMedGoogle Scholar
- Park SE, Pham DT, Boinett C, Wong VK, Pak GD, Panzner U, et al. The phylogeography and incidence of multi-drug resistant typhoid fever in sub-Saharan Africa. Nat Commun. 2018;9:5094. DOIPubMedGoogle Scholar
- Carey ME, Dyson ZA, Ingle DJ, Amir A, Aworh MK, Chattaway MA, et al.; Global Typhoid Genomics Consortium Group Authorship. Global diversity and antimicrobial resistance of typhoid fever pathogens: Insights from a meta-analysis of 13,000 Salmonella Typhi genomes. Elife. 2023;12:12. DOIPubMedGoogle Scholar
- Rao RS, Sundararaj T, Subramanian S, Shankar V, Murty SA, Kapoor SC. A study of drug resistance among Salmonella typhi and Salmonella paratyphi A in an endemic area, 1977-79. Trans R Soc Trop Med Hyg. 1981;75:21–4. DOIPubMedGoogle Scholar
- Ali A, Ali HA, Shah FH, Zahid A, Aslam H, Javed B. Pattern of antimicrobial drug resistance of Salmonella Typhi and Paratyphi A in a teaching hospital in Islamabad. J Pak Med Assoc. 2017;67:375–9.PubMedGoogle Scholar
- Irfan S, Hasan Z, Qamar F, Ghanchi N, Ashraf J, Kanji A, et al. Ceftriaxone resistant Salmonella enterica serovar Paratyphi A identified in a case of enteric fever: first case report from Pakistan. BMC Infect Dis. 2023;23:267. DOIPubMedGoogle Scholar
- Sajib MSI, Tanmoy AM, Hooda Y, Rahman H, Munira SJ, Sarkar A, et al. Trends in antimicrobial resistance amongst Salmonella Paratyphi A isolates in Bangladesh: 1999-2021. PLoS Negl Trop Dis. 2023;17:
e0011723 . DOIPubMedGoogle Scholar - Rahman SIA, Dyson ZA, Klemm EJ, Khanam F, Holt KE, Chowdhury EK, et al. Population structure and antimicrobial resistance patterns of Salmonella Typhi isolates in urban Dhaka, Bangladesh from 2004 to 2016. PLoS Negl Trop Dis. 2020;14:
e0008036 . DOIPubMedGoogle Scholar - Rahman SIA, Nguyen TNT, Khanam F, Thomson NR, Dyson ZA, Taylor-Brown A, et al. Genetic diversity of Salmonella Paratyphi A isolated from enteric fever patients in Bangladesh from 2008 to 2018. PLoS Negl Trop Dis. 2021;15:
e0009748 . DOIPubMedGoogle Scholar - Hooda Y, Sajib MSI, Rahman H, Luby SP, Bondy-Denomy J, Santosham M, et al. Molecular mechanism of azithromycin resistance among typhoidal Salmonella strains in Bangladesh identified through passive pediatric surveillance. PLoS Negl Trop Dis. 2019;13:
e0007868 . DOIPubMedGoogle Scholar - Molloy A, Nair S, Cooke FJ, Wain J, Farrington M, Lehner PJ, et al. First report of Salmonella enterica serotype paratyphi A azithromycin resistance leading to treatment failure. J Clin Microbiol. 2010;48:4655–7. DOIPubMedGoogle Scholar
- World Health Organization. Typbar-TCV [cited 2024 Jun 18]. https://extranet.who.int/prequal/vaccines/p/typbar-tcv
- World Health Organization. TYPHIBEV [cited 2024 Jun 18]. https://extranet.who.int/prequal/vaccines/p/typhibevr
- World Health Organization. SKYTyphoid [cited 2024 Jun 18]. https://extranet.who.int/prequal/vaccines/p/skytyphoid-multi-inj
- Typhoid Vaccine Acceleration Consortium. A third TCV receives WHO prequalification amidst rising rates of drug-resistant typhoid. 20 March 2024 [cited 2024 Jun 18]. https://www.coalitionagainsttyphoid.org/a-third-tcv-receives-who-prequalification-amidst-rising-rates-of-drug-resistant-typhoid
- Burki T. Typhoid conjugate vaccine gets WHO prequalification. Lancet Infect Dis. 2018;18:258. DOIPubMedGoogle Scholar
- Shakya M, Neuzil KM, Pollard AJ. Prospects of future typhoid and paratyphoid vaccines in endemic countries. J Infect Dis. 2021;224(Suppl 2):S770–4. DOIPubMedGoogle Scholar
- Qadri F, Khanam F, Liu X, Theiss-Nyland K, Biswas PK, Bhuiyan AI, et al. Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomised trial. Lancet. 2021;398:675–84. DOIPubMedGoogle Scholar
- Zaman K, Naser AM, Power M, Yaich M, Zhang L, Ginsburg AS, et al. Lot-to-lot consistency of live attenuated SA 14-14-2 Japanese encephalitis vaccine manufactured in a good manufacturing practice facility and non-inferiority with respect to an earlier product. Vaccine. 2014;32:6061–6. DOIPubMedGoogle Scholar
- Thorpe TC, Wilson ML, Turner JE, DiGuiseppi JL, Willert M, Mirrett S, et al. BacT/Alert: an automated colorimetric microbial detection system. J Clin Microbiol. 1990;28:1608–12. DOIPubMedGoogle Scholar
- Weinstein MP, Lewis JS II. The Clinical and Laboratory Standards Institute Subcommittee on antimicrobial susceptibility testing: background, organization, functions, and processes. J Clin Microbiol. 2020;58:e01864–19. DOIPubMedGoogle Scholar
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II—The design and analysis of cohort studies. IARC Sci Publ. 1987; (
82 ):1–406.PubMedGoogle Scholar - Carrasco JL. iccCounts: An R package to estimate the intraclass correlation coefficient for assessing agreement with count data. R J. 2022;14:229–43. DOIGoogle Scholar
- Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, et al.; GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:369–81. DOIPubMedGoogle Scholar
- Garrett DO, Longley AT, Aiemjoy K, Yousafzai MT, Hemlock C, Yu AT, et al. Incidence of typhoid and paratyphoid fever in Bangladesh, Nepal, and Pakistan: results of the Surveillance for Enteric Fever in Asia Project. Lancet Glob Health. 2022;10:e978–88. DOIPubMedGoogle Scholar
- Meiring JE, Shakya M, Khanam F, Voysey M, Phillips MT, Tonks S, et al.; STRATAA Study Group. Burden of enteric fever at three urban sites in Africa and Asia: a multicentre population-based study. Lancet Glob Health. 2021;9:e1688–96. DOIPubMedGoogle Scholar
- Shakya M, Voysey M, Theiss-Nyland K, Colin-Jones R, Pant D, Adhikari A, et al.; TyVAC Nepal Team. Efficacy of typhoid conjugate vaccine in Nepal: final results of a phase 3, randomised, controlled trial. Lancet Glob Health. 2021;9:e1561–8. DOIPubMedGoogle Scholar
- Patel PD, Liang Y, Meiring JE, Chasweka N, Patel P, Misiri T, et al.; TyVAC team. Efficacy of typhoid conjugate vaccine: final analysis of a 4-year, phase 3, randomised controlled trial in Malawian children. Lancet. 2024;403:459–68. DOIPubMedGoogle Scholar
- Dewan AM, Corner R, Hashizume M, Ongee ET. Typhoid Fever and its association with environmental factors in the Dhaka Metropolitan Area of Bangladesh: a spatial and time-series approach. PLoS Negl Trop Dis. 2013;7:
e1998 . DOIPubMedGoogle Scholar - Saad NJ, Lynch VD, Antillón M, Yang C, Crump JA, Pitzer VE. Seasonal dynamics of typhoid and paratyphoid fever. Sci Rep. 2018;8:6870. DOIPubMedGoogle Scholar
- Sur D, Ali M, von Seidlein L, Manna B, Deen JL, Acosta CJ, et al. Comparisons of predictors for typhoid and paratyphoid fever in Kolkata, India. BMC Public Health. 2007;7:289. DOIPubMedGoogle Scholar
- Tadesse BT, Khanam F, Ahmmed F, Liu X, Islam MT, Kim DR, et al. Association among household water, sanitation, and hygiene (WASH) status and typhoid risk in urban slums: prospective cohort study in Bangladesh. JMIR Public Health Surveill. 2023;9:
e41207 .PubMedGoogle Scholar - Kuehn R, Stoesser N, Eyre D, Darton TC, Basnyat B, Parry CM. Treatment of enteric fever (typhoid and paratyphoid fever) with cephalosporins. Cochrane Database Syst Rev. 2022;11:
CD010452 .PubMedGoogle Scholar - Browne AJ, Chipeta MG, Fell FJ, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, et al.; GRAM Typhoid Collaborators. Estimating the subnational prevalence of antimicrobial resistant Salmonella enterica serovars Typhi and Paratyphi A infections in 75 endemic countries, 1990-2019: a modelling study. Lancet Glob Health. 2024;12:e406–18. DOIPubMedGoogle Scholar
- Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, et al. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio. 2018;9:e00105–18. DOIPubMedGoogle Scholar
- Dong BQ, Yang J, Wang XY, Gong J, von Seidlein L, Wang ML, et al. Trends and disease burden of enteric fever in Guangxi province, China, 1994-2004. Bull World Health Organ. 2010;88:689–96. DOIPubMedGoogle Scholar
- Andrews JR, Qamar FN, Charles RC, Ryan ET. Extensively drug-resistant typhoid—are conjugate vaccines arriving just in time? N Engl J Med. 2018;379:1493–5. DOIPubMedGoogle Scholar
- Sahastrabuddhe S, Carbis R, Wierzba TF, Ochiai RL. Increasing rates of Salmonella Paratyphi A and the current status of its vaccine development. Expert Rev Vaccines. 2013;12:1021–31. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: September 08, 2025
1These first authors contributed equally to this article.
2Thes authors contributed as joint senior authors.
Table of Contents – Volume 31, Number 10—October 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sadia Isfat Ara Rahman, icddr,b, 68, Shaheed Tajuddin Ahmed Sarani, Mohakhali, Dhaka 1212, Bangladesh
Top