Volume 31, Number 7—July 2025
Research
Peromyscus spp. Deer Mice as Rodent Model of Acute Leptospirosis
Cite This Article
Citation for Media
Abstract
Leptospirosis is a global zoonotic disease affecting humans, wildlife, companion, and domestic animals. Incidental hosts can contract the disease directly or indirectly from asymptomatic reservoir hosts, most commonly small rodents. The Golden Syrian hamster is recognized as the dominant rodent model for acute leptospirosis because the animals are susceptible to many serovars and are used to maintain laboratory strains and test bacterin vaccine efficacy. However, hamsters are primarily used in survival-based studies, and investigations into host immune response and disease pathogenesis are limited. We found that Peromyscus leucopus white-footed deer mice are susceptible to acute leptospirosis, and thus might be an alternative rodent model. Furthermore, similar to hamsters, deer mice produce circulating foamy macrophages in response to Leptospira challenge. Deer mice exhibit differences in response to different serovars, clinical disease severity, kidney and liver lesions, and an overall sex effect, with male mice demonstrating more severe clinical signs and higher bacterial burden.
Leptospirosis is a global zoonosis causing severe disease in humans, domestic animals, and wildlife (1). In cattle and most livestock species, leptospirosis causes abortions and reproductive losses, resulting in serious veterinary health and economic concerns for producers (2,3). Commercial vaccines are available but are not cross-protective across Leptospira serogroups. Because of the range in clinical sign severity and the fastidious nature of culturing the pathogen, leptospirosis diagnosis can be challenging, and the disease is consistently underrecognized by clinicians. Leptospira spp. bacteria colonize the kidneys and are typically shed by reservoir hosts in their urine, where they are transmitted from the environment to incidental hosts (4). The primary reservoir hosts are members of the rodent family. Infected mice and rats are largely asymptomatic, and cohabitation in field environments, barns, and dwellings presents ample opportunity to foster transmission to humans and domestic animals.
The small animal models used to study acute leptospirosis are hamsters, guinea pigs, and gerbils (5–7). Although vaccines exist for many domestic species, commercial bacterins require validation before release. For those purposes, Golden Syrian hamsters (Mesocricetus auratus) are widely considered the standard model for leptospirosis research (8). Unlike most wildlife rodents, hamsters demonstrate acute and typically fatal signs of infection when challenged, although severity of disease can vary among Leptospira serovars, species, and strains (9–11). Hamsters are used to passage laboratory strains for research purposes, such as tests or the recovery of virulence (8). Similarly, hamsters are used in survival studies to test commercial bacterin vaccines (as required by the US Department of Agriculture), regardless of the intended host species (12). Although the research community relies heavily on the hamster model, it has notable limitations. They are mostly used for survival metrics instead of disease mechanism or immunology–based studies and suffer from a lack of reagents (such as markers of immune cell types) and resources (such as genomic/proteomic databases). Further, hamsters are largely not found in the wild and thus do not accurately model naturally occurring leptospirosis transmission.
Peromyscus mice, commonly known as deer mice, are phylogenetically more similar to hamsters in the Cricetidae family than are the classic Muridae Mus musculus mice (13,14). Wild deer mice, including a variety of subspecies (one of the most abundant being P. leucopus white-footed deer mice), are found in parts of North America and are used for bacterial and viral disease research, including research into Lyme disease, anaplasmosis, hantavirus (P. maniculatus mice), and viral encephalitis (15,16). For many of those diseases, deer mice serve as reservoir hosts and play crucial roles in tickborne disease life cycles and transmission kinetics, where they are characterized by a lack of clinical signs and reduced inflammatory immunological responses (16,17). Some aspects of the transmission and movement of strains of Leptospira from wildlife to livestock are unknown, and deer mice might serve as a potential environmental wildlife reservoir similar to other documented rodent hosts (18).
Our group strives to improve tools and metrics to assess immunological responses in rodent models that are key for leptospirosis research. Previously, we established that hamsters produce circulating foamy macrophages in response to Leptospira and bacillus Calmette-Guérin challenge, and the presence of those cell populations is correlated with disease severity (11,19,20). Foamy macrophages are best known for their characteristic appearance in the tissue of granulomas caused by Mycobacterium tuberculosis complex but are not typically found in circulation. Foamy macrophage vacuoles contain defined lipid droplets, but unlike the foamy macrophages that contain mycobacteria in tuberculous granulomas, those cells do not appear to contain leptospires in the challenged hamster model (11,21). In this study, we sought to assess the suitability of Peromyscus spp. mice as an alternative rodent model of leptospirosis.
Animals
We obtained P. leucopus deer mice 6–13 weeks of age from the LL stock Peromyscus Genetic Stock Center at the University of South Carolina (Columbia, SC, USA). All procedures and experiments were approved by the National Animal Disease Center Animal Care and Use Committee. Deer mice were monitored daily for health evaluation and had ad libitum access to food and water.
Experimental Design
This work includes data from 3 independent experiments. Leptospira borgpetersenii serogroup Ballum serovar Arborea strain LR131 and L. interrogans serogroup Canicola serovar Canicola strain LAD1 were propagated at 29°C in HAN media (22). Animals were challenged by intraperitoneal injection with 0.5 mL containing 1 × 107 leptospires (LR131 or LAD1) or 0.5 mL HAN media alone. Noninjection (negative) male and female control mice were used for nontreatment comparisons.
We monitored deer mice daily for clinical signs including lethargy; blood in urine; blood from nose, urogenital tract, or on paws; or lack of grooming/hygiene. We euthanized animals at the appearance of severe clinical signs such as blood from nose, paws, or urogenital tract or extreme lethargy (monitored by response to a human handler). We collected whole blood smears, kidney and liver samples for culture and quantitative PCR (qPCR) and tissues for histopathologic examination (spleen, lung, heart, kidney, liver, pancreas, and omental adipose).
Because severe clinical signs developed in male and female mice at different timepoints, we harvested control animals of the opposite sex as necessary. In a subset of female mice challenged with L. interrogans strain LAD1, acute signs of disease did not develop; we euthanized those mice ≈3 weeks postchallenge.
Culture and qPCR
For all challenged animals, we harvested a kidney or section of liver and macerated the specimen in 5 mL of HAN media containing 5-fluorouracil (100 µg/µL) for Leptospira culture. We used numerous dilutions of macerated suspensions to inoculate HAN media plus 5-fluorouracil as described previously (22) and incubated at 37°C in 5% CO2 (23). We determined bacterial burden of liver and kidney by using genomic lipL32 qPCR as previously reported (19).
Blood and Tissue Processing and Evaluation
We collected blood smear slides using the feathered edge technique, stained them with Giemsa solution, and evaluated them by microscopy. We performed a 100-cell differential count classifying the number of neutrophils, lymphocytes, monocytes, foamy macrophages, and combined eosinophils and basophils.
We took tissue samples of similar size and section for all groups from liver, kidney, spleen, lung, heart, and omental adipose tissue. We collected tissues into formalin and transferred to 70% ethanol after 24 hours. We used standard paraffin-embedding techniques to further process the fixed samples. We transferred cut paraffin-embedded tissues sections (4-μm-thick sections) to Superfrost Plus charged microscope slides (Thermo Fisher Scientific, https://www.thermofisher.com), after which we stained them with hematoxylin and eosin. We evaluated histologic sections by light microscopy using an Olympus B41 microscope and DP 23 Olympus camera and captured images with cellSens Olympus software (Evident Scientific, https://evidentscientific.com).
We performed immunohistochemistry with the Ventana Ultra Discovery on representative formalin-fixed, paraffin-embedded tissue sections 4 μm thick. We deparaffinized sections in Discovery Wash (Roche, https://www.roche.com) and achieved antigen retrieval by incubation with cell-conditioning solution 1, a citrate-based buffer (pH 6.0). We performed a blocking step with Discovery Goat Ig Block (Roche) for 20 minutes at 35°C. We incubated sections for 32 minutes at 35°C with primary antibody against LipL32 at 1:100 dilution in Discovery antibody diluent (Roche). The signal was detected with rabbit Dako EnVision-HRP system (Agilent, https://www.agilent.com) for 32 minutes at 35°C and visualized with 3,3′-diaminobenzidine (Roche). We counterstained the sections with Harris Hematoxylin (Leica Biosystems, https://www.leicabiosystems.com).
Statistics
We evaluated stained whole-blood smear cell counts (cell numbers/100 leukocytes) using simple linear regression models in R version 4.1.0 (The R Project for Statistical Computing, https://www.r-project.org). We evaluated each cell type independently (lymphocytes, neutrophils, monocytes, foamy macrophages, and combined eosinophils and basophils). For bacterial burden data, we evaluated genomic lipL32 qPCR independently between kidney and liver. For all, each group was fit as a fixed effect detailing male mice with acute disease, female mice with acute disease, and time-matched nonclinical control female mice (showing no signs of disease) taken when acute male mice reached endpoint. We included 2 control groups, media alone and noninjection negative controls. We evaluated specific contrasts within cell types with pairwise contrasts. We determined significance by a p value of <0.05; error bars represent SEs. We analyzed Spearman correlations in R between foamy macrophage counts and bacterial burden of liver and kidney across all deer mice.
We challenged acclimated male and female Peromyscus spp. deer mice with L. interrogans serogroup Canicola serovar Canicola (strain LAD1) and L. borgpetersenii serogroup Ballum serovar Arborea (strain LR131) and evaluated both media alone and noninjection controls. Deer mice showed severe clinical signs of disease within the first week postchallenge, establishing them as an alternative rodent model of acute leptospirosis (Figure 1). Although both L. borgpetersenii and L. interrogans strains caused acute disease, endpoint separation between male and female mice is striking and demonstrates that male mice were more susceptible than female mice to both LR131 and LAD1 (p<0.01 between sexes for both) (Figure 1). Because male mice were more susceptible, we also evaluated a control group of nonclinical female mice not displaying severe signs of disease but euthanized at the same time as acutely ill male mice.
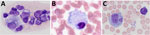
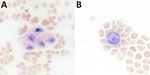
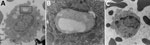
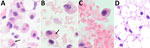
Previously, our group described the unique production of circulating foamy macrophages by hamsters challenged with Leptospira (11), a feature not replicated in experimentally infected laboratory mice, guinea pigs, or rats (E. Putz et al., unpub. data). By examining Giemsa-stained slides of whole blood smears, we identified circulating foamy macrophages, consistent with those described in hamsters, in deer mice (Table; Figure 2). Intracellular lipid droplets are defining features of foamy macrophages. We confirmed the presence of those lipids with microscopy techniques including Oil Red O staining (Figure 3) and transmission electron microscopy (Figure 4). Similar to findings in the hamster model, no leptospires were detectable within foamy macrophages by transmission electron microscopy (24). Additional hematologic findings included neutrophilia and lymphopenia (Table).
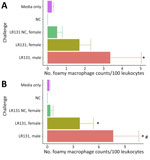
Substantial populations of foamy macrophages were found in the blood of deer mice challenged with both L. borgpetersenii and L. interrogans (Figure 5). Control noninjected animals did not produce any foamy macrophages, but media alone controls had sparse production of an occasional foamy-filled cell, suggesting a possible immune response to HAN media. LAD1-challenged male mice produced significantly more foamy macrophages than did media alone (p<0.01) and negative control (p = 0.02) animals. Male LAD1-infected deer mice also produced more foamy macrophages (3.36 + 1.16/100 counted leukocytes) than did acute female (1.70 + 1.37/100 counted leukocytes; p = 0.22) or control female counterparts (0.50 + 2.17/100 counted leukocytes; p = 0.12), but differences were not significant (Figure 5, panel A). Differences among challenged groups were stronger for L. borgpetersenii LR131–infected animals, whereas male deer mice generated significantly greater numbers of foamy macrophages than did acute female (p = 0.05), nonclinical control female (p = 0.01), HAN media alone (p<0.01), or negative control (p<0.01) groups (Figure 5, panel B). Given that LR131-challenged deer mice reached endpoints more quickly than LAD1-challenged deer mice, foamy macrophage cell percentages appear to be associated with disease severity. This finding further corroborates the sex effect seen in this study; higher foamy macrophage proportions were found in male mice than in acutely diseased female mice (Figure 5).
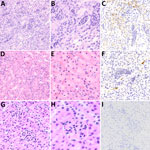
No significant histologic findings were present in the HAN media only and negative control groups. Challenge groups for strain LR131 and strain LAD1 had variable and nonspecific changes in the spleen and lung. The spleen had variable lymphoid follicle expansion, which can result from antigenic stimulation, and extramedullary hematopoiesis. The lung had variable hemorrhage and congestion; some vessels contained neutrophils, attributed to the peripheral blood neutrophilia.
Both strains LR131 and LAD1 kidney-associated omental adipose tissue had hemorrhage and inflammatory cell infiltrates when associated with kidney inflammatory lesions. Cell infiltrates consisted of variable numbers of neutrophils, lymphocytes, macrophages, and suspected foamy macrophages (Figure 6). Some macrophages, or possible foamy macrophages, contained erythrocytes (Figure 6), also seen in the blood (Figure 2). Immunohistochemistry also confirmed the presence of leptospires within kidney adipose tissue (Appendix Figure).
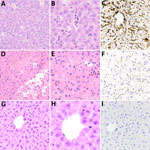
Significant lesions of interest were present in the kidney and liver and varied between challenge strains; inflammatory lesions in the kidney were more severe and diffuse in a higher number of deer mice challenged with strain LR131 than those challenged with LAD1 (Figure 7). The LR131-challenged acute disease group had renal cortex intratubular neutrophils that extended into the medulla, tubular necrosis, interstitial neutrophilic nephritis, hemorrhage, and perivascular neutrophils and lymphocytes (Figure 7). The LAD1-challenged acute disease group had similar renal lesions, but they were less severe and diffuse, and hemorrhage and medullary inflammatory infiltrates were rare. Lesion severity differences were further supported by immunohistochemistry, which demonstrated a greater number of leptospires in LR131-infected mice (Figure 7).
In the histologic assessment of the liver, animals infected with strain LR131 had mild hepatic lesions consisting of intrasinusoidal neutrophils, possibly associated with peripheral neutrophils, and low numbers of occasional perivascular lymphocytes (Figure 8). This finding contrasted with the LAD1-challenged group, which had mild to severe diffuse liver lesions (Figure 8). The contrasting severity of lesions and density of leptospires differences were confirmed by immunohistochemistry (Figure 8). Milder lesions consisted of hepatocyte vacuolar change, hepatocyte swelling (cell edema), and a few areas of perivascular lymphocytic inflammation. Severe liver lesions consisted of hepatocyte dissociation and purulent hepatocyte necrosis that was multifocal to diffuse with a midzonal to centrilobular pattern, often bridging (Figure 8).
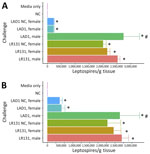
Culture was positive in liver and kidney samples from all challenged animals, and we quantified bacterial load by using genomic equivalent qPCR for lipl32. Bacterial loads were similar between challenges, but the highest levels of leptospires per gram were seen in the liver (Figure 9). For both Leptospira species–infected deer mice, bacterial load was higher in kidney and liver samples from male mice than in samples from acute female, nonclinical female, HAN media alone, and negative control groups (Figure 9, panels A, B) (p<0.01). As seen previously in hamsters (25), between LAD1- and LR131-challenged female mice, bacterial burdens were higher in both liver and kidney samples for L. borgpetersenii challenge than L. interrogans (p<0.01). In contrast, bacterial load in male mice did not differ in kidney (p = 0.56) or liver (p = 0.33) specimens between challenge strains (Figure 9). Foamy macrophage percentages were significantly correlated with bacterial load of leptospires in liver and kidney specimens (p<0.01), further demonstrating their association with virulence.
Acute disease developed in P. leucopus deer mice in response to Leptospira challenge, offering an alternative acute rodent model to elucidate pathogenic mechanisms of leptospirosis. From an evolutionary standpoint, deer mice are more closely related to hamsters than to M. musculus mice (13,14). In addition to displaying severe clinical signs such as hematuria, blood on nose and foot pads, and lethargy, deer mice produced foamy macrophages in response to both L. interrogans and L. borgpetersenii challenge, which correlated with disease severity. Our group previously identified that hamsters produce circulating foamy macrophages in response to bacterial infection, but in our studies, those cells do not appear in mouse, guinea pig, or rat models of leptospirosis. Because circulating foamy macrophages are present in low numbers and can be unevenly distributed in blood smears, we found visual examination of the entire blood smear (versus a 100-cell count differential) was sometimes needed to detect foamy macrophages. Also, foamy macrophages can be missed if only automated analyzer evaluation is used for hematology analysis (with specific parameters flagged to require visual examination, as can occur in human medicine). This work suggests that circulating foamy macrophages might be limited to the Cricetidae family, but evaluation of additional rodent species, further investigation in Peromyscus subspecies, and review of blood smears from other species susceptible to leptospirosis would be necessary to confirm this unique finding.
Peromyscus spp. mice are commonly recognized as asymptomatic reservoir hosts, particularly for the Lyme disease bacteria Borrelia burgdorferi. Much work has been done exploring the reservoir host components and pathogen interactions between Peromyscus species and other rodents. Gene expression work suggests Peromyscus spp. mice respond differently than M. musculus mice to lipopolysaccharide challenge (17,26) and have altered immune cell interactions, such as largely inactive or altered immunoglobulin gamma Fc receptors (27). Previous work in deer mice has established that Peromyscus transcriptomic profiles are more aligned with M2 macrophage than M1 polarization in response to endotoxin (26) and are associated with alternatively activated monocytes (17). Specifically, deer mice showed a low inducible nitric oxide synthase 2 (Nos2), high arginase 1 (Arg1) expression ratio in response to lipopolysaccharide challenge (28), which is typically associated with a damage repair or antiinflammatory response. Such nonclassical activation profiles could contribute to the production of foamy macrophages, but the mechanism of circulating foamy macrophage formation and their functional capabilities are still unclear. Implications of foamy macrophages for host immune response and bacterial persistence are also of interest, because the acquisition of low-density lipoprotein cholesterol within foamy macrophage vacuoles is viewed as a nutrient source for pathogens (29). Of note, foamy macrophages have been tissue-associated with other infectious agents, such as Chlamydia spp. and Toxoplasma spp., so the association with foamy macrophages might not be leptospirosis-specific (29). However, Leptospira infection in hamsters, and now deer mice, is to our knowledge the only model to produce foamy macrophages in circulation. A small portion of foamy macrophages were produced in a minority of deer mice injected with HAN media absent of leptospires. Although the mechanism behind foamy macrophage formation remains unknown in the context of leptospirosis, HAN media is rich in fatty acids and nutrients (22), and some level of immune activation from the intraperitoneal challenge could conceivably have resulted in rare circulating foamy macrophages.
In cases of human leptospirosis, primary kidney lesions of acute tubular necrosis and acute interstitial nephritis are observed, which were also seen in deer mice histology. Both LR131- and LAD1-challenged deer mice had acute nephritis, primarily affecting the renal tubules and with more severe lesions in LR131-challenged tissues. Renal failure might have resulted in the LR131-infected animals reaching severe endpoints before their LAD1-infected counterparts. On histologic sections, suspected foamy macrophages in challenged deer mice were only associated with renal omentum. When conducting hematoxylin and eosin staining on deer mice samples in this study, identifying foamy macrophages was more complex than in hamsters because the clear round vacuoles associated with lipid content were also associated with smaller foamy diffuse vacuoles and, sometimes, erythrophagocytosis. Histologic findings of foamy macrophages, macrophages with cytoplasmic vacuoles, and the erythrophagocytosis in either macrophages or foamy macrophage tended to overlap. Collectively, this finding suggests that foamy macrophages might phagocytose erythrocytes (30).
Human leptospirosis patients who experience Weil’s syndrome have a high mortality rate (≈10%) because of renal and hepatic failure (31) associated with direct damage of hepatocytes and Kupffer cells (32,33). LAD1-challenged deer mice had lesions that ranged from mild hepatocyte swelling (cell edema) with vacuolar hepatopathy, often in a centrilobular pattern, to severe necrotizing hepatitis with a midzonal to centrilobular pattern. The milder lesions of hepatocyte vacuolar change and cell swelling are not specific and appeared more consistent with the foamy cytoplasm of hydropic degeneration that can result from hypoxia, anorexia, or altered metabolic or nutritional states. Alternatively, those lesions might precede the more advanced necrotizing hepatitis, considering clinical signs ranged from absent to mild in the LAD1-challenged deer mice with milder hepatic lesions.
Bacterial burden data illustrate high pathogen loads in major organs of interest; the highest burden of leptospires per gram was found in liver tissue, not kidney tissue (Figure 9). That finding is consistent with pathogenesis tracking of acutely ill hamsters challenged with L. interrogans, where heavy dissemination to immune responsive organs is apparent (34). Over time in nonfatal conditions, host immune responses control leptospires in most systems, whereas persistent colonization of the kidneys enables persistent shedding. Most notable differences in burden data are reflected in the significantly reduced bacterial load in female LAD1-challenged animals and female controls (Figure 9). That finding supports the sex effect differences reported but also suggests that reduced disease severity in those animals is associated with reduced leptospire presence in liver and kidneys. Although unsurprising, the same was not true of LR131-challenged female mice and female controls, suggesting L. interrogans and L. borgpetersenii pathogenesis and interactions with host sex could be different. Although a Leptospira dose response was not evaluated in this study, follow-up research with the concurrent investigation of alternative routes of inoculum administration, such as conjunctival challenge, could be warranted.
Sex effects are associated with leptospirosis in humans and animals, including hamsters, and consistently favor more severe disease in male infectees (35–37). We found the same; male deer mice succumbed more quickly to severe disease endpoints (Figure 1), produced more circulating foamy macrophages (Figure 5), and harbored higher bacterial burdens in kidney and liver samples (Figure 9). Of note, Borrelia infection studies in P. leucopus mice show no difference between male and female littermates (28), but other work illustrates older male deer mice caught in the wild were more likely to harbor tick infestation (38). The mechanism of the leptospirosis sex effect is still unknown, but work with hamsters in leishmaniasis showed that treating female hamsters with testosterone increased the size of lesions (39), suggesting androgens might play a role in pathogenesis. In other rodent models of bacterial disease with sex effects, sex differences of Listeria monocytogenes in mice (more resistance in males) found that more severe disease was associated with increased levels of interleukin-10 (IL-10), and IL-10 knockout mice showed no differences between sexes (40). Of additional relevance, M2 macrophages can secrete IL-10, illustrating another immune component that should be investigated in the deer mouse model of leptospirosis.
In conclusion, our results demonstrate that deer mice are susceptible to acute leptospirosis. Ultimately, an alternative rodent model for acute disease is valuable. Unlike hamsters, deer mice are found readily in the wild; additional field work should survey leptospirosis prevalence among those populations. The role of circulating foamy macrophages in the pathogenesis of leptospirosis is yet to be elucidated but currently appears limited to the Cricetidae family. Collectively, this work demonstrates that deer mice represent a possible real-world host that could enable further modeling of transmission kinetics and pathogenesis of leptospirosis.
Dr. Putz is a research microbiologist specializing in zoonotic bacterial diseases within the Infectious Bacterial Research Unit at the National Animal Disease Center in Ames, Iowa. She and her team use multifaceted approaches, such as microscopy, transcriptomics, and immunological investigation, to investigate host and pathogen interactions and response to bacterial infection, principally leptospirosis, brucellosis, and tuberculosis.
Acknowledgments
We extend our immense appreciation to Denise Chapman, Jean Kaptur, and the National Animal Disease Center vivarium animal care staff for the care and handling of animals.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendations or endorsement by the US Department of Agriculture (USDA). USDA is an equal opportunity provider and employer. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA or the Agricultural Research Service. USDA was the sole funder for this research.
References
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:
e0003898 . DOIPubMedGoogle Scholar - Loureiro AP, Lilenbaum W. Genital bovine leptospirosis: A new look for an old disease. Theriogenology. 2020;141:41–7. DOIPubMedGoogle Scholar
- Martins G, Lilenbaum W. Control of bovine leptospirosis: Aspects for consideration in a tropical environment. Res Vet Sci. 2017;112:156–60. DOIPubMedGoogle Scholar
- Putz EJ, Nally JE. Investigating the immunological and biological equilibrium of reservoir hosts and pathogenic Leptospira: balancing the solution to an acute problem? Front Microbiol. 2020;11:2005. DOIPubMedGoogle Scholar
- Zuerner RL, Alt DP, Palmer MV. Development of chronic and acute golden Syrian hamster infection models with Leptospira borgpetersenii serovar Hardjo. Vet Pathol. 2012;49:403–11. DOIPubMedGoogle Scholar
- Nally JE, Chantranuwat C, Wu XY, Fishbein MC, Pereira MM, Da Silva JJ, et al. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am J Pathol. 2004;164:1115–27. DOIPubMedGoogle Scholar
- Yukawa M, Mochizuki K, Imamura S. Susceptibility of Mongolian gerbils (Meriones unguiculatus) to leptospires and the protective effect of vaccination. Vet Microbiol. 1990;24:63–71. DOIPubMedGoogle Scholar
- Walker A, Srinivas GB. Opportunities and strategies to further reduce animal use for Leptospira vaccine potency testing. Biologicals. 2013;41:332–7. DOIPubMedGoogle Scholar
- Putz EJ, Sivasankaran SK, Fernandes LGV, Brunelle B, Lippolis JD, Alt DP, et al. Distinct transcriptional profiles of Leptospira borgpetersenii serovar Hardjo strains JB197 and HB203 cultured at different temperatures. PLoS Negl Trop Dis. 2021;15:
e0009320 . DOIPubMedGoogle Scholar - Randall R, Cooper HK. The golden hamster (Cricetus auratus) as a test animal for the diagnosis of leptospirosis. Science. 1944;100:133–4. DOIPubMedGoogle Scholar
- Putz EJ, Andreasen CB, Stasko JA, Fernandes LGV, Palmer MV, Rauh MJ, et al. Circulating foamy macrophages in the Golden Syrian hamster (Mesocricetus auratus) model of leptospirosis. J Comp Pathol. 2021;189:98–109. DOIPubMedGoogle Scholar
- Srinivas GB, Walker A, Rippke B. USDA regulatory guidelines and practices for veterinary Leptospira vaccine potency testing. Biologicals. 2013;41:298–302. DOIPubMedGoogle Scholar
- Steppan S, Adkins R, Anderson J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol. 2004;53:533–53. DOIPubMedGoogle Scholar
- Barbour AG, Shao H, Cook VJ, Baldwin-Brown J, Tsao JI, Long AD. Genomes, expression profiles, and diversity of mitochondria of the White-footed Deermouse Peromyscus leucopus, reservoir of Lyme disease and other zoonoses. Sci Rep. 2019;9:17618. DOIPubMedGoogle Scholar
- Yawitz TA, Barts N, Kohl KD. Comparative digestive morphology and physiology of five species of Peromyscus under controlled environment and diet. Comp Biochem Physiol A Mol Integr Physiol. 2022;271:
111265 . DOIPubMedGoogle Scholar - Barbour AG. Infection resistance and tolerance in Peromyscus spp., natural reservoirs of microbes that are virulent for humans. Semin Cell Dev Biol. 2017;61:115–22. DOIPubMedGoogle Scholar
- Milovic A, Duong JV, Barbour AG. The infection-tolerant white-footed deermouse tempers interferon responses to endotoxin in comparison to the mouse and rat. eLife. 2024;12:12. DOIPubMedGoogle Scholar
- Caraballo L, Rangel Y, Reyna-Bello A, Muñoz M, Figueroa-Espinosa R, Sanz-Rodriguez CE, et al. Outbreak of intermediate species Leptospira venezuelensis spread by rodents to cows and humans in L. interrogans–endemic region, Venezuela. Emerg Infect Dis. 2024;30:1514–22. DOIPubMedGoogle Scholar
- Fernandes LGV, Putz EJ, Stasko J, Lippolis JD, Nascimento ALTO, Nally JE. Evaluation of LipL32 and LigA/LigB knockdown mutants in Leptospira interrogans serovar Copenhageni: impacts to proteome and virulence. Front Microbiol. 2022;12:
799012 . DOIPubMedGoogle Scholar - Putz EJ, Andreasen CB, Stasko JA, Hamond C, Olsen SC, Nally JE, et al. Circulating foamy macrophages and other features of bacillus Calmette-Guérin challenge in Golden Syrian hamsters. Vaccine. 2025;55:
127037 . DOIPubMedGoogle Scholar - Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–8. DOIPubMedGoogle Scholar
- Hornsby RL, Alt DP, Nally JE. Isolation and propagation of leptospires at 37 °C directly from the mammalian host. Sci Rep. 2020;10:9620. DOIPubMedGoogle Scholar
- Hamond C, Adam EN, Stone NE, LeCount K, Anderson T, Putz EJ, et al. Identification of equine mares as reservoir hosts for pathogenic species of Leptospira. Front Vet Sci. 2024;11:
1346713 . DOIPubMedGoogle Scholar - Putz EJ, Andreasen CB, Stasko JA, Fernandes LGV, Palmer MV, Rauh MJ, et al. Circulating foamy macrophages in the Golden Syrian hamster (Mesocricetus auratus) model of leptospirosis. J Comp Pathol. 2021;189:98–109. DOIPubMedGoogle Scholar
- Fernandes LGV, Hamond C, Tibbs-Cortes BW, Putz EJ, Olsen SC, Palmer MV, et al. CRISPR-prime editing, a versatile genetic tool to create specific mutations with a single nucleotide resolution in Leptospira. MBio. 2024;15:
e0151624 . DOIPubMedGoogle Scholar - Balderrama-Gutierrez G, Milovic A, Cook VJ, Islam MN, Zhang Y, Kiaris H, et al. An infection-tolerant mammalian reservoir for several zoonotic agents broadly counters the inflammatory effects of endotoxin. MBio. 2021;12:12. DOIPubMedGoogle Scholar
- Barbour AG, Duong JV, Long AD. Lyme disease agent reservoirs Peromyscus leucopus and P. maniculatus have natively inactivated genes for the high-affinity immunoglobulin gamma Fc Receptor I (CD64). Pathogens. 2023;12:12. DOIPubMedGoogle Scholar
- Cook V, Barbour AG. Broad diversity of host responses of the white-footed mouse Peromyscus leucopus to Borrelia infection and antigens. Ticks Tick Borne Dis. 2015;6:549–58. DOIPubMedGoogle Scholar
- Guerrini V, Gennaro ML. Foam cells: one size doesn’t fit all. Trends Immunol. 2019;40:1163–79. DOIPubMedGoogle Scholar
- Wang W, Liu W, Fidler T, Wang Y, Tang Y, Woods B, et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2V617F mice. Circ Res. 2018;123:e35–47. DOIPubMedGoogle Scholar
- Petakh P, Isevych V, Kamyshnyi A, Oksenych V. Weil’s disease-immunopathogenesis, multiple organ failure, and potential role of gut microbiota. Biomolecules. 2022;12:12. DOIPubMedGoogle Scholar
- De Brito T, Silva AMGD, Abreu PAE. Pathology and pathogenesis of human leptospirosis: a commented review. Rev Inst Med Trop São Paulo. 2018;60:
e23 . DOIPubMedGoogle Scholar - Miyahara S, Saito M, Kanemaru T, Villanueva SY, Gloriani NG, Yoshida S. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil’s disease. Int J Exp Pathol. 2014;95:271–81. DOIPubMedGoogle Scholar
- Philip N, Priya SP, Jumah Badawi AH, Mohd Izhar MH, Mohtarrudin N, Tengku Ibrahim TA, et al. Pulmonary haemorrhage as the earliest sign of severe leptospirosis in hamster model challenged with Leptospira interrogans strain HP358. PLoS Negl Trop Dis. 2022;16:
e0010409 . DOIPubMedGoogle Scholar - Jansen A, Stark K, Schneider T, Schöneberg I. Sex differences in clinical leptospirosis in Germany: 1997-2005. Clin Infect Dis. 2007;44:e69–72. DOIPubMedGoogle Scholar
- Traxler RM, Callinan LS, Holman RC, Steiner C, Guerra MA. Leptospirosis-associated hospitalizations, United States, 1998-2009. Emerg Infect Dis. 2014;20:1273–9. DOIPubMedGoogle Scholar
- Tomizawa R, Sugiyama H, Sato R, Ohnishi M, Koizumi N. Male-specific pulmonary hemorrhage and cytokine gene expression in golden hamster in early-phase Leptospira interrogans serovar Hebdomadis infection. Microb Pathog. 2017;111:33–40. DOIPubMedGoogle Scholar
- Poje JE, Azevedo JF, Nair N, Mahachi K, Frank LE, Sherpa P, et al. Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) infection prevalence and host associations of ticks found on Peromyscus spp. in Maryland. J Med Entomol. 2022;59:752–7. DOIPubMedGoogle Scholar
- Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect Immun. 2002;70:2288–96. DOIPubMedGoogle Scholar
- Pasche B, Kalaydjiev S, Franz TJ, Kremmer E, Gailus-Durner V, Fuchs H, et al. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun. 2005;73:5952–60. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: June 12, 2025
Table of Contents – Volume 31, Number 7—July 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ellie J. Putz, National Animal Disease Center, USDA Agricultural Research Service, 1920 Dayton Ave, Ames, IA 50010, USA
Top