Volume 31, Number 7—July 2025
Research
Peromyscus spp. Deer Mice as Rodent Model of Acute Leptospirosis
Figure 7
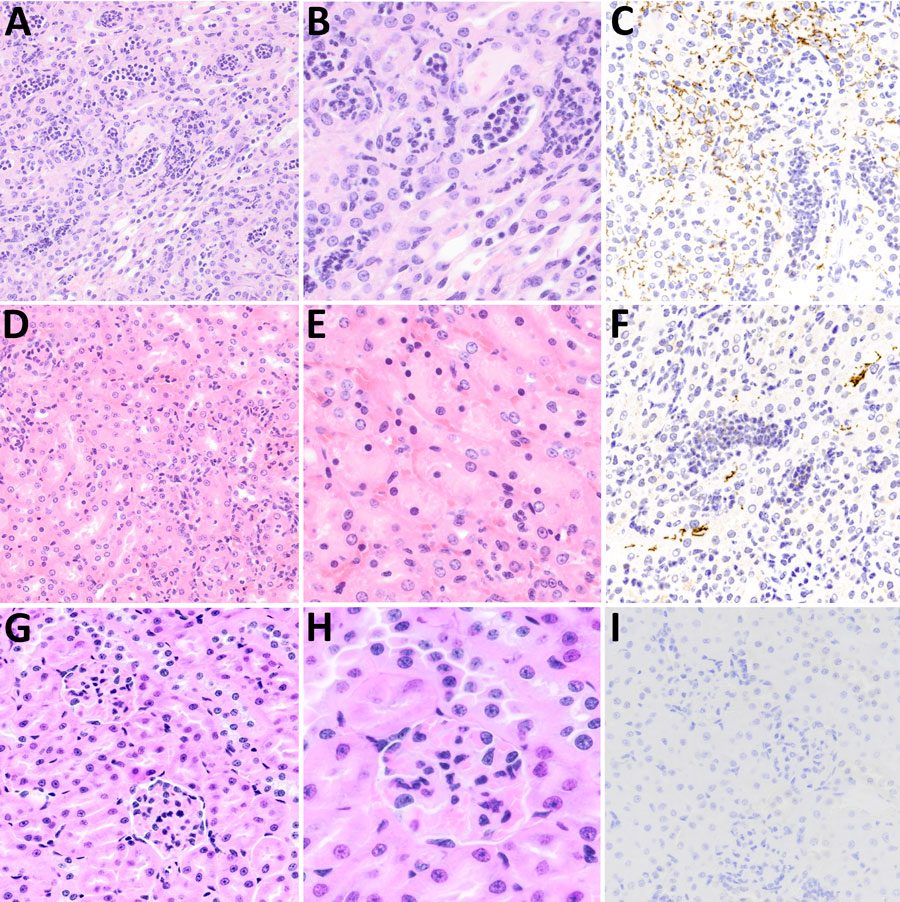
Figure 7. Microscopic and immunohistochemical examination of kidney samples taken from challenged and control deer mice in study of Peromyscus species deer mice as rodent model of acute leptospirosis. A–C) Samples taken from LR131-challenged deer mice showing acute diffuse purulent nephritis and nephrosis. The renal cortex and medulla appeared similar with severe diffuse intratubular neutrophilic inflammation, loss and necrosis of tubular lining cells, and resulting nephrosis. Intertubular neutrophilic infiltrates in the interstitium and some neutrophilic glomerular infiltrates (not common) were present. There were scattered areas of hemorrhage (not pictured). A, B) Hematoxylin and eosin–stained tissue (A, original magnification ×20; B, original magnification ×40); C) LipL32 immunohistochemistry-labeled kidney (original magnification ×20). D–F) Samples taken from LAD1-challenged deer mice showing most severe renal lesion associated with LAD1. Examination showed acute multifocal purulent nephritis and nephrosis with lymphocytic and slightly purulent interstitial nephritis. The renal cortex contained multifocal intratubular neutrophilic inflammation, loss and necrosis of tubular lining cells with the accumulation of eosinophilic material and resulting nephrosis. Intertubular lymphocytes and fewer neutrophils were scattered in the interstitium. D, E) Hematoxylin and eosin–stained tissue (D, original magnification ×20; E, original magnification ×40); F) LipL32 immunohistochemistry-labeled kidney (original magnification ×20). G–I) Sample of noninfected control animal kidney. G) Hematoxylin and eosin–stained tissue (G, original magnification ×20; H, original magnification ×40); I) LipL32 immunohistochemistry negative (original magnification ×20).